Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility tests are used to determine the type and quantity of antimicrobial agents used in chemotherapy. One of the most important functions of a clinical laboratory is to determine the antimicrobial susceptibility.
Antimicrobial susceptibility of pathogens refers to the limitation of pathogens to grow in the presence of effective antibiotics. There are two methods that can be used to determine the susceptibility of a potential pathogen to antimicrobial agents. They are:
- Disk diffusion method
- Tube dilution method
Disc Diffusion Method (Kirby – Bauer Test)
William Kirby and Alfred Bauer, in 1966 first introduced the principle of measuring zones of inhibition around antibiotic discs to determine antimicrobial agent susceptibilities. It is a rapid, convenient method to determine the susceptibilities of microorganisms to antimicrobial agents and a most common procedure used in susceptibility testing in clinical laboratory.
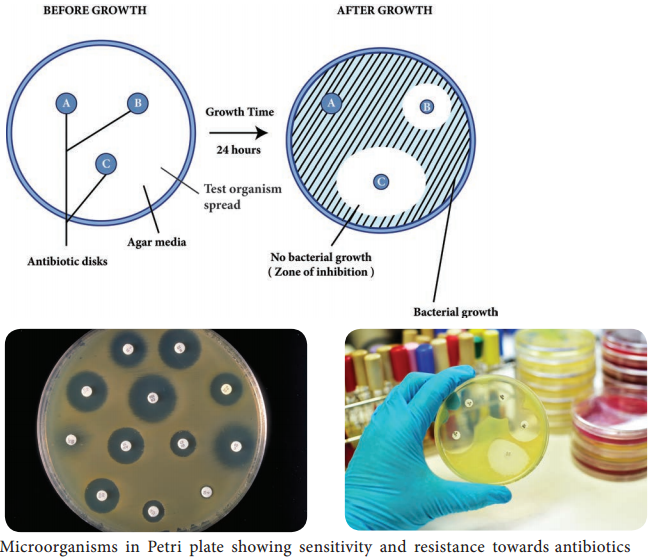
Filter paper discs containing known concentrations of antimicrobial agents are placed onto the surface of an agar plate (Muller – Hinton agar medium) inoculated with the test bacterium (Figure 3.3). The plate is incubated for 16 to 18 hours, and the zones of inhibition are read around each paper disc. During the incubation periods, the antimicrobial agent diffuses through the agar, and a concentration gradient of agent is established.
At some point in this gradient, growth of the susceptible bacteria is suppressed, and no growth is observed within a circular zone around disc. The size of a zone of inhibition must be compared to a standard Table for that particular drug before accurate comparisons can be made.
Thus, enabling to classify pathogens as susceptible (S), intermediate or resistant (R) to a drug. The procedure is highly regulated and controlled by the clinical and laboratory standards institute (CLSI) and must be accompanied by a rigorous quality assurance program including performance by certified and/or licensed personnel when the results are to be reported in clinical settings.
Minimal Inhibitory Concentration (MIC) Test
The potency of an effective antimicrobial agent is expressed in terms of minimal inhibitory concentration (MIC). It is the minimum concentration of drug that will inhibit the growth of pathogen. The MIC is determined by serial dilutions of antimicrobial agents in tubes with standard amount of bacteria. Turbidity (cloudiness) after incubation indicates bacterial growth and lack of turbidity indicates that the growth of bacteria is inhibited.
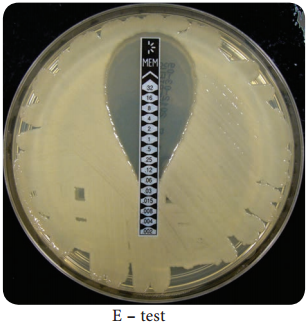
E – test
This is another test to determine the minimum inhibitory concentration where a plastic strip containing a gradient of the antimicrobial agent is used (Figure 3.4). An elliptical zone of inhibitory concentration can be noted with the help of a scale printed on the strip.
The Minimal Bactericidal Concentration (MBC) Test
MBC test is similar to MIC, the minimal bactericidal concentration test is used to determine the amount of antimicrobial agent required to rather kill the pathogen. In MBC test, samples taken from MIC tubes are transferred to drug free plates. Bacterial growth in these subcultures indicates that some bacterial cells have survived antimicrobial drug. The lowest concentration of drug for which no growth occurs is the minimum bactericidal concentration.
The tube dilution method is considered accurate for determining susceptibility of a pathogen to precise quantities of antimicrobial agent. However, the method is time consuming, expensive, and not practical for use in most clinical laboratories for routine susceptibility testing.