NCERT Solutions for Class 12 Chemistry Chapter 6 contains concept wise details of the chapter. The solutions help the students prepare well for the examination. The diagrammatic representations and step wise solutions make it easy for the students to understand. Also the answers are accurate and are provided by the subject matter experts.
NCERT Solutions are provided for reference in various boards (CBSE, MP board, UP board, Gujarat board). The students appearing for these boards and competitive exams can score well by referring to the NCERT Solutions.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | Chemistry |
| Chapter | Chapter 6 |
| Chapter Name | General Principles and Processes of Isolation of Elements |
| Number of Questions Solved | 31 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements
Class 12 Chemistry chapter 6 explains the applications of various metals in our day-to-day life. Different terminologies such as refining, calcination, concentration, benefaction, roasting, etc. are very well explained here. The concepts of thermodynamics for the extraction of copper, aluminium and zinc are also mentioned here.
This chapter contains the details of various chemical processes associated with metallurgy. Being an important chapter, it will help the students score well during the examinations.
NCERT IN-TEXT QUESTIONS
Question 1.
Name some ores which can be concentrated by magnetic separation method.
Answer:
Only those ores can be concentrated by magnetic separation method in which either the ore particles or the impurities associated with it are of magnetic nature. For example, ores of iron such haematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) are magnetic and can be concentrated by this method. Similarly, casseterite (SnO2) an ore of tin is non-magnetic while the impurities of tungstates of iron and chromium are of magnetic nature. Magnetic separation is effective in this case also.
Question 2.
What is the significance of leaching in the extraction of aluminium?
Answer:
Aluminium contains silica (SiO2), iron oxide (Fe2O3) and titanium oxide (TiO4) as impurities. These impurities can be removed by the process of leaching. During leaching, the powdered bauxite ore is heated with a concentrated (45%) solution of NaOH at 473-523 K, where alumina dissolves as sodium meta-aluminate and silica as sodium silicate leaving Fe2O3, TiO2 and other impurities behind:
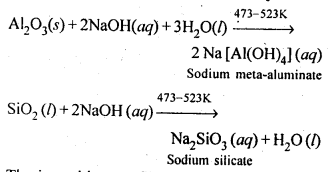
The impurities are filtered off and solution of sodium meta-aluminate is neutralised by passing CO2 when hydrated alumina separates out while sodium silicate remains in solution. The hydrated aluminathus obtained is filtered, dried and heated to give back pure alumina.
![]()
Thus, by leaching, pure alumina can be obtained from bauxite ore.
Question 3.
The reaction : Cr2O2(s) + 2Al(s) → Al2O3(s) + 2Cr(s) ; ∆G° = – 421 kJ is thermodynamically feasible as is apparent from the value of ∆G°. Why does not it take place at room temperature ?
Answer:
Though the reaction is feasible, it does not proceed at room temperature because all the reactants and products are solids. At elevated temperature, when chromium starts melting, the reaction becomes feasible.
Question 4
Is it true that under certain conditions, Mg can reduce Al2O3 and Al can reduce MgO ? What are those conditions ?
Answer:
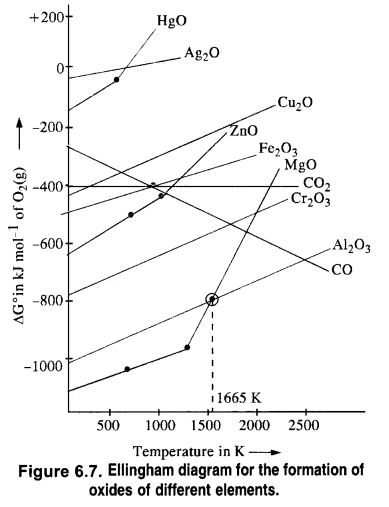
If we look at the Ellingham diagram, it becomes evident that the plots for Al and Mg cross each other at 1350 °C (1623 K). Below this temperature, Mg can reduce Al2O3 and above this temperature, Al can reduce MgO.
NCERT EXERCISE
Question 1.
Copper can be extracted by hydrometallurgy but not zinc. Explain.
Answer:
Copper is a comparatively less active metal as its reduction potential i.e. E° (Cu2+/Cu) is high (+0-34V). It can be displaced from a solution of Cu2+ ions by more active metals which have E° value lower than copper. For example, E° of zinc (Zn2+/Zn) is -0.76V and thus, zinc can displace copper from the solution of Cu2+ ions. In contrast, to displace zinc from a solution of Zn2+ ions a more reactive metal than zinc is required like, Na, K, Mg, Ca, etc. But, the more active metals readily react with water forming their corresponding ions and evolve hydrogen gas.
[2Na + 2H2O → 2NaOH + H2],
Thus, it is difficult to displace zinc from a solution of Zn2+ ions. Elance, copper can be extracted by hydrometallurgy but not zinc.
Question 2.
What is the role of the depressant in the froth floatation process?
Answer:
A depressant suppresses the formation of froth with a particular compound in the froth floatation process by reacting chemically with it. Thus, it helps in the separation of two metal sulphides present together in a particular ore.
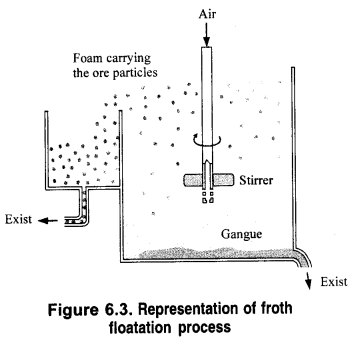
In actual process, the sulphide ore is finely powdered and is mixed with water to form a slurry in a tank as shown in Fig. 6.3. To this oily component of the sulphide ore particles by water. As a result, ore and oil constitute hydrophobic or water-repelling components while gangue and water form a lyophilic or water-attracting component.
Question 3.
Why is the extraction of copper from its sulphide ore difficult than that from its oxide through reduction?
Answer:
Sulphide ore of copper (Cu2S) cannot be directly reduced by either coke or hydrogen because ∆fG° of Cu2S is more than those of CS2 and H2S that will be formed as a result of the reaction.
These reactions are, therefore, not feasible. However, the ∆fG° of Cu2O is lower than that of CO2. Therefore, the sulphide ore is first roasted to Cu2O which is then reduced.

Question 4.
Explain
(i) zone refining
(ii) column chromatography. (C.B.S.E. Sample Paper 2015)
Answer:
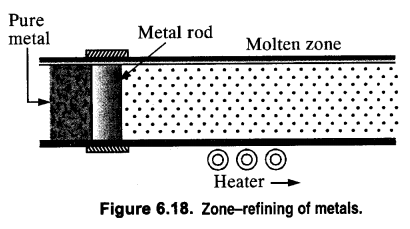
(i) Zone refining (Fractional Crystallisation). This method is used only if a metal in almost pure state is required. Metals like germanium and gallium which are used in semiconductors are purified by this method. The principle of zone refining is based on the fact that impurities are more soluble in molten metal than in solid metal.
(ii) Chromatographic method. This method can also be employed on small scale for the purification of certain metals. Adsorption chromatography is normally used for this purpose. Different components present in a given sample are adsorbed to a different extent on the surface of the adsorbent.
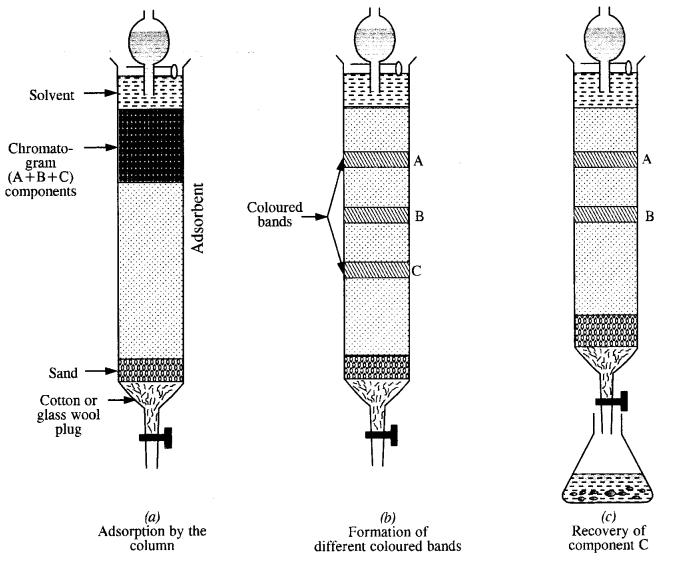
Question 5.
Out of C and CO, which is a better reducing agent at 673 K?
Answer:
When carbon reacts with dioxygen, two reactions are possible
C(s) + O2(g) → CO2(g) ……(i)
2C(s) + O2(g) → 2CO(g) …..(ii)
When CO is used as a reducing agent, it gets oxidized to CO2
2CO + O2 → 2CO2 …(iii)
It is clear from the Ellingham diagram that at 673K the ∆G° for the oxidation of CO to CO2 is more negative than the reaction (i) and reaction (ii). Therefore, CO is a better reducing agent than C. It is supported by the fact that the curve for the reaction (iii) lies below the curve for the reaction (i) and reaction (ii) at 673K. An element below in the Ellingham diagram reduces the oxide of other metal which lies above it.
Question 6.
Name the common elements present in the anode mud in electrolytic refining of copper. Why are they so present?
Answer:
The common elements present in the anode mud are antimony, selenium, tellurium, silver, gold, and platinum. These elements settle down under anode as anode mud because they are less reactive and are not effected by CuSO4 – H2SO4solution.
Question 7.
Write the chemical reactions which take place in different zones in the blast furnace during the extraction of iron.
Answer:
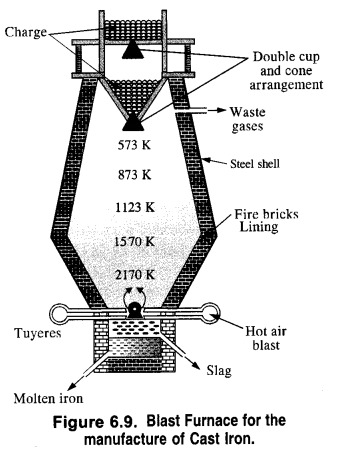
The chemical reactions which take place in the blast furnace are briefly discussed.
Zone of combustion. At the bottom of the furnace, the blast of hot air causes the combustion of coke into carbon dioxide. The reaction is highly exothermic and a temperature of nearly 2170 K develops. It supplies most of the heat required for the process.
![]()
Zone of heat absorption. As CO2 gas rises up, it combines with more coke to form carbon monoxide. Since the reaction is endothermic, the temperature in the middle of the furnace is nearly 1570 K. It further decreases as the reaction proceeds.
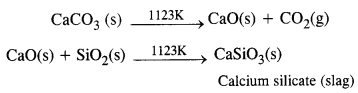
Zone of slag formation. In the middle of the furnace, the temperature is nearly 1123 K. Here limestone (CaCO3) decomposes to form CaO and CO2. The former (CaO) combines with silica (SiO2) which is an impurity in the haematite ore to form calcium silicate (CaSiO3) that is fusible. This implies that CaO has acted as a basic flux. It has combined with the acidic impurity of Si02 to form slag.
Zone of reduction: This is the upper part of the furnace. The temperature ranges between 500K to 900K. Here haematite (Fe2O3) is reduced to FeO.
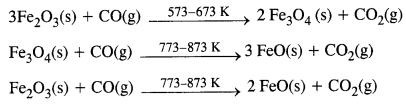
Further reduction of FeO to Fe occurs at higher temperatures (1123 K) by CO gas.
![]()
The direct reduction of iron ore left unreacted also occurs with carbon above 1123 K
![]()
Question 8.
Write the chemical reactions which take place in the extraction of zinc from zinc blende.
Answer:
Zinc blende is chemically zinc sulphide (ZnS). It undergoes the following reactions:
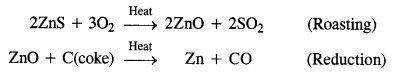
Zinc metal is in crude or impure form. It can be refined with the help of electro-refining. In this method, impure zinc is made anode while a plate of pure metal acts as the cathode. The electrolyte is aqueous zinc sulphate containing a small amount of dilute H2SO4. On passing electric current, Zn2+ ions from the electrolyte migrate towards the cathode and are reduced to zinc metal which gets deposited on the cathode.
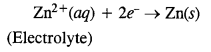
From anode, an equivalent amount of zinc gets oxidised to Zn2+ ions which migrate to the electrolyte
![]()
Question 9.
State the role of silica in the metallurgy of copper.
Answer:
During roasting, copper pyrites are converted into a mixture of FeO and Cu20. Thus, acidic flux silica is added during smelting to remove FeO (basic). FeO combines with SiO2to form famous silicate (FeSiO3) slag which floats over molten matte.
Question 10.
What is meant by the term chromatography?
Answer:
The term chromatography was originally derived from the Greek word chroma meaning colour and graphy meaning writing because the method was first used for the separation of coloured substances (plant pigments) into individual components. Now the term chromatography has lost its original meaning and the method is widely used for separation, purification and characterization of the components of a mixture whether coloured or colourless.
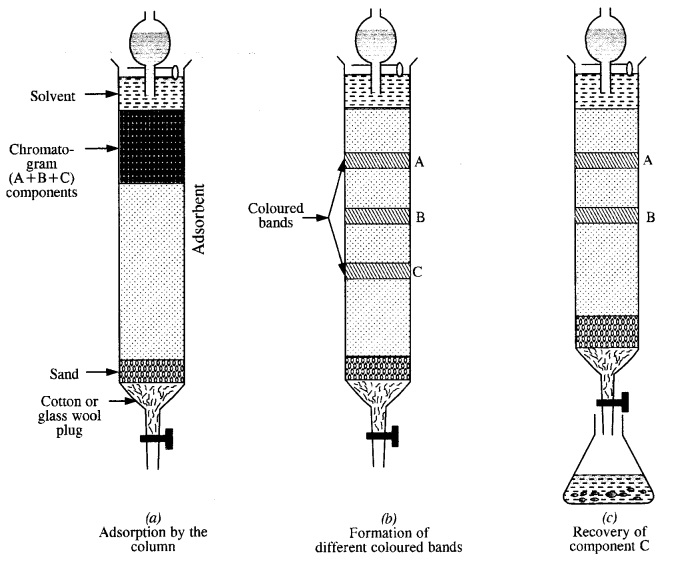
Question 11.
What criterion is followed for the selection of the stationary phase in chromatography?
Answer:
The stationary phase is selected in such a way that it is capable of adsorbing the impurities more strongly than the elements to be purified. Under this condition, the impurities are retained by stationary phase i. e„ these cannot be eluted easily while the pure component, which is weakly adsorbed is easily eluted.
Question 12.
Describe a method for the refining of nickel.
Answer:
Nickel is refined by Mond’s process.
Mond’s process. Mond’s process is used for the refining of nickel metal. In the process, impure metal is heated in a current of carbon monoxide (CO) at 330 to 350 K to form nickel carbonyl which is of volatile nature. The vapours of the metal carbonyl escape leaving behind impurities. Upon heating to about 450 K, nickel carbonyl decomposes to give pure nickel.
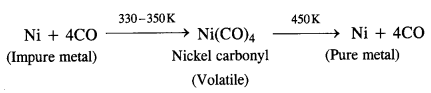
From the above discussion, we may conclude that the basic requirements for the refining of metal by Vand Alkel process and Mond’s process are:
- The metal should form a volatile compound with the available reagent.
- The volatile compound should be easily decomposable so that metal in pure form may be regenerated.
Question 13.
How can you separate alumina from silica in bauxite ore associated with silica? Give equations if any.
Answer:
The purification of bauxite containing silica as the main impurity is done by Serpeck’s process. The powdered ore is mixed with coke and heated to about 2073 K in an atmosphere of nitrogen. Silica (SiO2) is reduced to silicon which being volatile escapes. Alumina (Al2O3) is converted into aluminium nitride (AIN) by reacting with nitrogen. It is hydrolysed upon heating with water to get the precipitate of Al(OH)3. From the precipitate, Al2O3 is recovered upon strong heating.
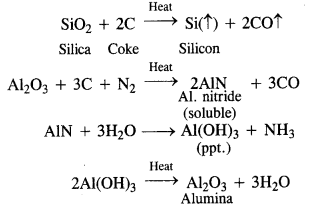
Question 14.
Giving examples, differentiate between calcination and roasting.
Answer:

Question 15.
How is ‘cast iron’ different from ‘pig iron’?
Answer:
Iron obtained from the blast furnace is called pig iron. It contains about 4% carbon and other impurities of S, P, Si, Mn etc. When pig iron is mixed with scrap iron and coke and then heated in a blast of hot air, some impurities are removed. Cast iron is obtained. It contains 3% carbon and some other impurities. It is hard and brittle.
Question 16.
Differentiate between mineral and ore.
Answer:
The naturally occurring chemical substances in form of which the metal occurs in the earth along with impurities are called minerals. The minerals from which the metal can be extracted conveniently and economically are called ore. Thus, all ores are minerals but all minerals are not ores. For example, iron is found in the earth’s crust oxides, carbonates and sulphides. Out of these minerals of iron, the oxides of iron are employed for the extraction of the metal. Therefore, oxides of iron are the ores of iron. Similarly, aluminium occurs in the earth’s crust in form of two minerals, i.e, bauxite (Al2O3. x H2O) and clay (Al2O3.2SiO. 2H2O). Out of these two minerals, Al can be conveniently and economically extracted from bauxite the ore of alluminium.
Question 17.
Why is copper matte put in the silicon-lined converter?
Answer:
Copper matte consists of a mixture of Cu2S and Cu2O. Along with that, it also contains a small amount of FeS and FeO. It is put in a silicon-lined converter known as a Bessemer converter. Some silica (SiO2) is also added and a blast of hot air is blown. As a result, a number of reactions take place.
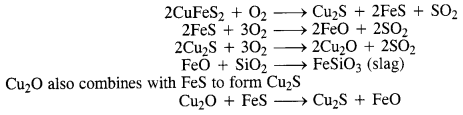
Question 18.
What is the role of cryolite in the metallurgy of aluminium?
Answer:
The role of cryolite is two-fold
- It makes alumina a good conductor of electricity.
- It lowers the fusion (melting point) temperature of both from 2323K to about 1140K.
Question 19.
How is leaching carried out in case of low-grade copper?
Answer:
Leaching in case of low-grade copper is carried out by reacting with an acid like H2SO4 in the presence of air when copper is oxidised to Cu2+ ions which pass into the solution. For example.
2 Cu(s) + 2 H2SO4 (aq) + O2 (g) → 2CuSO4 (aq) + 2 H2O(l)
or Cu + 2H+ (aq) + 1/2 O2(g) → Cu2+ (aq) + H2O(l)
Question 20.
Why is zinc not extracted from zinc oxide through reduction using CO?
Answer:
The standard free energy of formation (Δf G°) of CO2 from CO (fig 6.8) is higher than that of the formation-of ZnO from Zn. Therefore, CO cannot be used to reduce ZnO to Zn.
Question 21.
The value of ∆fG° for the formation of Cr2O3 is – 540 kJ mol-1 and that of Al2O3 is – 827 kJ mol-1. Is the reduction of Cr2O3 with Al possible? (Pb. Board2009)
Answer:
The two thermochemical equations may be written as follows :
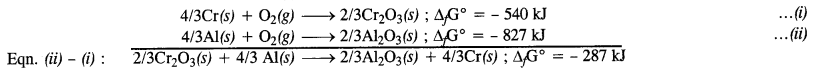
Since ∆G° comes out to be negative, the reaction is feasible.
Question 22.
Out of C and CO, which is a better reducing agent for ZnO?
Answer:
The Δf G° of CO2 from CO is always a higher reduction of ZnO to Zn. In contrast, Δf G° of CO from C is lower at a temperature above 1180K while that of CO2 from C is lower at temperatures above 1270K than Δf G° of ZnO. Thus above 1270K, ZnO can be reduced to Zn by C. In actual practice, the reduction is usually carried out around 1673K. Thus out of C and CO, C is a better reducing agent than CO for ZnO.
Question 23.
The choice of a reducing agent in a particular case depends on the thermodynamic factors. How do you agree with this statement? Support your opinion with two examples.
Answer:
Thermodynamic factors have a major role in selecting the reducing agent for a particular reaction.
(i) Only that reagent will be preferred which will lead to decrease in free energy (∆G°) at a certain specific temperature.
(ii) A metal oxide placed lower in the Ellingham diagram cannot be reduced by the metal involved in the formation of the oxide placed higher in the diagram.
Examples:
(0 Al2O3 cannot be reduced by Cr present in Cr2O3 since the curve for Al2O3 is placed below that of Cr2O3 in the Ellingham diagram
(ii) CO cannot reduce ZnO because there is hardly any change in free energy (∆G°) as a result of the reaction.
Question 24.
Name the processes from which chlorine is obtained as the by-product. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Answer:
Chlorine is obtained as the by-product in the manufacture of sodium by Down’s process in which molten sodium chloride is subjected to electrolysis.
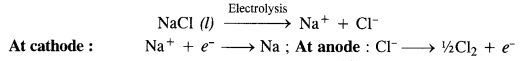
Sodium obtained by this method is almost pure while chlorine is the by-product.
Chlorine can also be obtained by carrying out the electrolysis of an aqueous solution of sodium chloride. The process is carried in Nelson’s cell. The various reactions which take place are as follows :
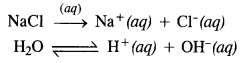
At cathode. Both Na+ and H+ ions migrate towards the cathode but H+ ions are discharged in preference to Na+ ions since their discharge potential is less. Na+ ions remain in the solution.
![]()
At anode: Both Cl– and OH– ions migrate towards the anode but Cl– ions are discharged in preference to OH” since their discharge potential is less. OH– ions remain in the solution.
![]()
Thus, in the electrolysis of an aqueous NaCl solution, H2 gas is evolved at the cathode and chlorine at the anode. The solution contains NaOH and is, therefore, basic in nature.
It is always better to prepare chlorine by Down’s process.
Question 25.
What is the role of a graphite rod in the electrometallurgy of aluminium?
Answer:
In the electrometallurgy of aluminium, oxygen gas is evolved at the anode. It reacts with graphite or carbon (graphite electrodes) to form carbon monoxide and carbon dioxide. In case some other metal electrodes act as an anode, then oxygen will react with aluminium formed during the process to form aluminium oxide (Al2O3) which will pass into the reaction mixture. Since graphite is cheaper than aluminium, its wastage or consumption can be tolerated.
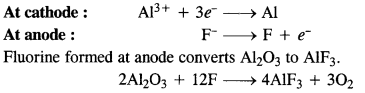
Question 26.
Outline the principles of refining of metals by the following methods:
(i) Zone refining
(ii) Electrolytic refining
(iii) Vapour phase refining
Answer:
(i) 1. This method is based on the principle that the impurities are more soluble in the molten state of metal (the melt) than in the solid-state. In the process of zone refining, a circular mobile heater is fixed at one end of a rod of impure metal. As the heater moves the molten zone of the rod also moves with it. As a result, pure metal crystallizes out of the melt and the impurities pass onto the adjacent molten zone. This process is repeated several times, which leads to the segregation of impurities at one end of the rod. Then the end with impurities is cut off. Silicon, boron, gallium, indium, etc can be purified by this process.
2. Column chromatography is a technique used to separate components of a mixture where components are in minute quantities. In chromatography, there are two phases: the mobile phase and the stationary phase. The stationary phase is immobile and immiscible. Al2O3 column is usually used as the stationary phase in column chromatography. The mobile phase may be a gas, liquid, or supercritical fluid in which the sample extracts dissolve. Then the mobile phase is forced to wave through the stationary phase. The component that is more strongly absorbed on the column takes a long time to travel than the component weakly, absorbed.
(ii) Electrolytic refining is the process of refining impure metal by using electricity. In this process, impure metal is made the anode and a strip of pure metal is made the cathode. A solution of a soluble salt of the same metal is taken as the electrolyte. When an electric current is passed, metal ions from the electrolyte are deposited at the cathode as pure metal and the impure metal from the anode dissolves into the electrolyte in the form options. The impurities present in the impure metal gets collected below the anode. This is called anode mud.
Anode : M → Mn++ ne–
Cathode : Mn+ + ne– → M.
(iii) Vapour phase refining is the process of refining metal by converting it into its volatile compound and then, decomposing is to obtain a pure metal to carry out this process.
(a) The metal should form a volatile compound with available reagent, and
(b) The volatile compound should be easily decomposable so that the metal can be easily recovered.
Nickel, Zirconium, and titanium are refined using this method.
Question 27.
Predict the conditions under which aluminium can be expected to reduce magnesium oxide.
Answer:
The equations for the formation of the two oxides are :
![]()
If we look at the plots for the formation of the two oxides on the Ellingham diagram, we find that they intersect at a certain point. The corresponding value of ∆G° becomes zero for the reduction of MgO by Al metal.
![]()
This means that the reduction of MgO by A1 metal can occur below this temperature.
Aluminium (Al) metal can reduce MgO to Mg above this temperature because ∆fG for Al2O3 is less as compared to that of MgO.
NCERT Solutions for Class 12 Chemistry Chapter6 General Principles and Processes of Isolation of Elements 30![]()
We hope the NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements help you. If you have any query regarding NCERT Solutions for Class 12 Chemistry Chapter 6 General Principles and Processes of Isolation of Elements, drop a comment below and we will get back to you at the earliest.