CBSE Sample Papers for Class 9 Science Paper 2 are part of CBSE Sample Papers for Class 9 Science. Here we have given CBSE Sample Papers for Class 9 Science Paper 2.
CBSE Sample Papers for Class 9 Science Paper 2
| Board | CBSE |
| Class | IX |
| Subject | Science |
| Sample Paper Set | Paper 2 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 9 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme as prescribed by the CBSE is given here. Paper 2 of Solved CBSE Sample Paper for Class 9 Science is given below with free PDF download solutions.
Time Allowed : 3 Hours
Max. Marks: 80
General Instructions
- The question paper comprises of two Sections, A and B. You are to attempt both the sections.
- All questions are compulsory. However an internal choice will be provided in two questions of 3 marks each and one question of five marks.
- All questions of Section A and all questions of Section B are to be attempted separately.
- Question numbers 1 to 2 in Section A are one-mark questions. These are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section A are two-marks questions. These are to be answered in about 30 words each.
- Question numbers 6 to 15 in Section A are three-marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section A are five-marks questions. These are to be answered in about 70 words each.
- Question numbers 22 to 27 in Section B are two-marks questions based on practical skills. These are to be answered in brief
Questions
SECTION-A
Question 1.
Name two greenhouse gases.
Question 2.
Sponges belong to invertebrates, give two peculiar characteristics of sponges.
Question 3.
An element X has atomic number 17, find its valency and electronic configuration.
Question 4.
Write four main symptoms of jaundice or hepatitis.
Question 5.
Give four properties of sound waves.
Question 6.
Name three subatomic particles of atom and give the difference between them.
OR
Calculate the number of aluminum ions in 0.051 g of Al2O3
[Atomic mass of Al = 27 u, O = 16 u, NA = 6.022 × 1023 mol-1]
Question 7.
What is saturated solution? How can you make the saturated solution unsaturated?
Question 8.
Give the cause, symptoms and control measures for malaria.
Question 9.
Sachin and Dhoni are friends and they study in the same class. Suhaas brings home made food as lunch whereas Sachin eats Burger, chips and French fries during Lunch Break from the school canteen. During a routine health checkup in the school, Doctor finds Sachin overweight to his age. Both Sachin and Dhoni have read that being overweight leads to diabetes and cardiovascular diseases. Both of them now want to help other students so that no other student could suffer from being overweight.
- What values are shown by Sachin and Dhoni?
- What should Sachin do now to keep his weight under control?
- How can these two boys help other students?
Question 10.
Name the organelle responsible for the removal of wastes and old worn out cells and why?
Question 11.
A ship sends ultrasonic waves that returns from the sea bed and is detected after 2.80 s. If the speed of ultrasound through sea water is 1531 m/s, what is the distance of the seabed from the ship?
Question 12.
- Why is it difficult to the hold a school bag having strap made of thin and strong string?
- Why are the walls of dam broader at the base and narrow at the top?
OR
What are fluids? How does upthrust exerted by a fluid on an object immersed in it vary with density of fluid?
Question 13.
- An object of mass 15 kg is moving with a uniform velocity of 4 m s-1. What is the kinetic energy possessed by the object?
- Does the transfer of energy take place when you push a huge rock with all your force and fail to move it? Where is the energy you spend, going?
Question 14.
(a) A light object and heavy object have same momentum, find out the ratio of their kinetic energies. Which one has larger kinetic energy?
(b) Name the energy present in a person who is parachuting.
Question 15.
A spoonful of sugar is added to a beaker containing 100 ml of water and stirred for a while. State any two observations that you will make.
Account your observation.
Question 16.
What are the causes, symptoms and methods of prevention and cure of AIDS?
Question 17.
With the help of a diagram show,
(a) Nitrogen cycle in nature
(b) Describe briefly any two processes involved in the cycling of nitrogen in the nature.
Question 18.
Four men lift a 250 kg box to a height of 1 m and hold it without raising or lowering it.
- How much work is done by the four men in lifting the box?
- How much work do they do in just holding it?
- Why do they get tired while holding it? (g = 10 m/s2)
- If the relative density of a body is 6.2, what will be its density in SI unit?
Question 19.
What do you mean by work? Give an example of negative work done? What is the work done in increasing the velocity of a car from 18 km/hr to 90 km/hr, if mass of the car is 2000 kg.
Question 20.
- State two factors on which the magnitude of buoyant force acting on a body immersed in a fluid depends.
- Will the buoyant force exerted by a liquid increase, if its volume is increased?
- Name the devices based on Archimedes principle.
Question 21.
Explain how animals in Vertebrata are classified into further subgroups.
OR
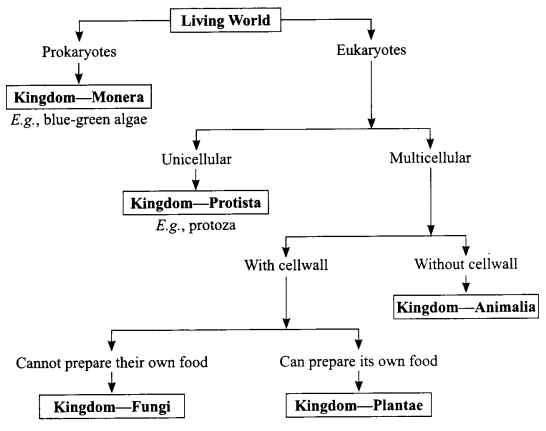
Give the hierarchy of 5 kingdom classification of living world.
SECTION-B
Question 22.
In an experiment 14 g of sodium hydrogen carbonate was allowed to react with 10 g of acetic acid. After the reaction was completed it was found that only 16.67 g solution was left. What is the cause of producing less amount of product?
Question 23.
Name different parts of neuron.
Question 24.
Give two functions of osmosis.
Question 25.
To make a solution, 40 g of common salt is dissolved in 280 g of water. Calculate its concentration in terms of mass by mass percentage of the solution.
Question 26.
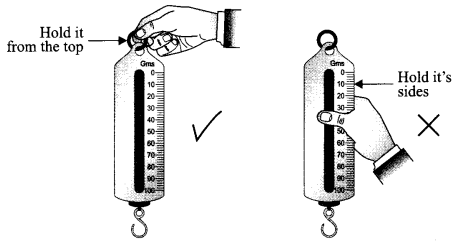
What precautions will you take while holding the spring balance to find the weight of a body?
Question 27.
In a chemical reaction 0.096 g of boron combines with 0.144 g of oxygen to form a compound.
Calculate the mass of the compound formed.
Answers
SECTION-A
Answer 1.
Methane and carbon dioxide
Answer 2.
- Presence of pores like ostia and osculum
- Presence of canal system
Answer 3.
Electronic configuration is 2, 8, 7. Valency is equal to 1.
Answer 4.
Symptoms of jaundice: High temperature, headache, joint pain, loss of appetite, dark yellow urine and irritating rashes, (any four)
Answer 5.
Properties of sound waves:
- These are longitudinal waves.
- These are produced by vibrations.
- These are mechanical waves.
- Their speed is least in gases and maximum in solids.
Answer 6.
Subatomic particles of atoms are electrons, protons and neutrons.
Electron:
- It is negatively charged.
- Its absolute mass is equal to 9.1 × 10-31 kg.
Proton:
- It is positively charged.
- Its absolute mass is equal to 1.67 × 10-28 kg.
Neutron:
- It is neutral.
- Its mass is slightly more than that of protons. Its absolute mass is equal to 1.675 × 10-28 kg.
OR

Answer 7.
When no more solute can be dissolved in a solvent at a given temperature, the solution is said to be a saturated solution. The same solution on heating/increasing its temperature can become unsaturated as the particles in the solution on heating gains energy and move apart from each other, thereby allowing some more solute particles to dissolve in it.
Answer 8.
Malaria is a common disease in our country particularly in the rural areas.
- Cause: It is caused by a protozoan parasite, plasmodium which gets injected into the blood of a healthy person when bitten by an infected female Anopheles mosquito.
- Symptoms: Headache, nausea and muscular pain.
High fever with shivering – this occurs periodically. - Control measures: Malaria can be controlled by a drug called quinine and its variants. Efforts are being made for developing antimalarial vaccine.
Answer 9.
- The values shown by Dhoni and Sachin are love for the fellow students and concern for the society.
- Sachin should avoid eating junk food like burger, pizza, chips etc. immediately.
- Dhoni and Sachin can do the following to help other students:
- They can request the school authority to keep only healthy food to be served in the school canteen.
- They can start a poster campaign in the school notice-board highlighting the ill effects of junk food.
Answer 10.
Lysosomes
They are capable of breaking down all organic material and keep the cell clear by digesting the worn out cells and cell organelles. They are membrane bound sacs with powerful digestive enzymes. When the cell is worn out and need to be destroyed, the lysosomes burst and the enzymes digests the cell.
Answer 11.
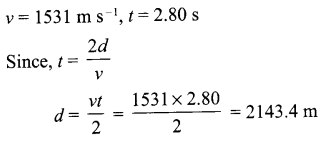
Answer 12.
- A thin and strong string has less surface area, due to which it exerts greater pressure on the shoulder of the person holding the bag using it. Thus, it becomes difficult to hold it.
- The pressure of water at the bottom is maximum. To withstand this pressure the walls at the bottom are made wider. On the contrary, pressure of water at top is comparatively minimum and thus, its wall are made narrower.
OR
- All liquids and gases are referred as fluids.
- Greater the density of the fluid, more is the upthrust.
- Lesser the density of the fluid, lesser is the upthrust.
Answer 13.
- K.E = \(\frac { 1 }{ 2 } \) mv2 = \(\frac { 1 }{ 2 } \) × 15 × (4)2 = 120 J
- Yes, the energy is used up in causing muscular contraction and is dissipated in the form of heat also.
Answer 14.
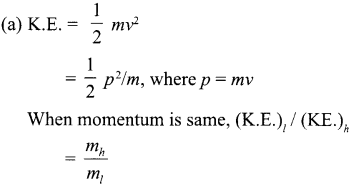
Lighter mass will have larger kinetic energy, since mH > mi.
(b) The parachutist will have kinetic energy and gravitational potential energy.
Answer 15.
Observations:
- Sugar disappears in water.
- The volume of water does not increase.
This is because there is lot of space between the water molecules and the added sugar molecules will occupy the space between the water molecules.
Answer 16.
Causes: AIDS is caused by retro-virus HIV (Human Immuno Deficiency Virus).
Mode of transmission: AIDS is transmitted from an infected person to a healthy person through sexual contact, blood transfusion, use of contaminated needle, from infected mother to the foetus.
Symptoms: The major symptoms of AIDS are –
- Swollen lymph nodes.
- Decreased Count of blood platelets causing haemorrhage, fever, patient becomes susceptible to other infections too due to breakdown of the immune system.
- Sweating at night and weight loss.
- Severe damage to brain which may lead to loss of memory, ability to speak and think.
Prevention: AIDS can be prevented by adopting the following precautions –
- Sexual contact with unknown person should be avoided.
- Transfusion of infected blood should be avoided. The blood donor should be tested for HIV negative.
- Disposable syringes and needles should be used.
- Common razor at the barber shop should be avoided.
Cure: No effective treatment for AIDS is available.
Answer 17.
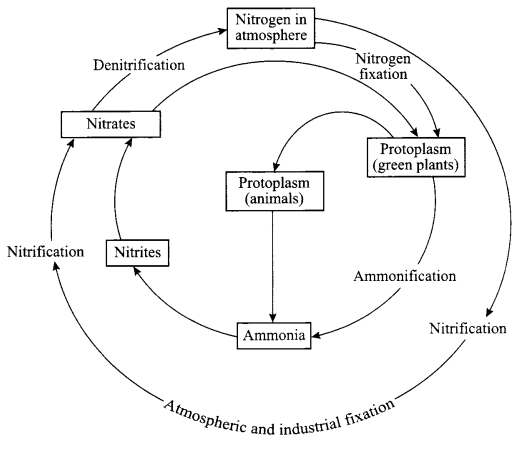
Fixing of nitrogen by nitrogen fixing bacteria which are found in root nodules of legumes or which are free living, ammonification by bacteria in the soil, conversion of ammonia to nitrites or nitrites to nitrates by different type of bacteria and then nitrates to nitrogen in air by different bacteria.
Answer 18.
- Work done in lifting
mgh = 250 × 10 × 1
= 2500 Joule % - Work done is zero as displacement is zero.
- Muscular energy is used up in holding, so they get tired on just holding.
- 6.2 × 103 kg/m3
Answer 19.
Work is said to be done when the force applied on an object moves it in its own direction.
Example of negative work-when a body is sliding on a surface, work done by the force of friction is negative.
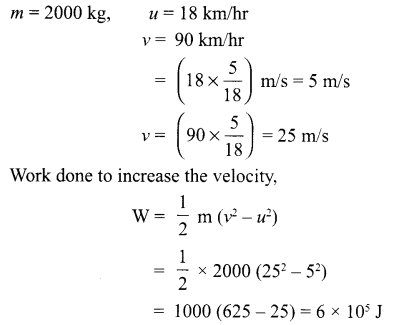
Answer 20.
- Buoyant force = Weight of liquid displaced
= (Volume of the liquid displaced) × (Density of liquid) × (Acceleration due to gravity)
So, density of liquid and volume of the liquid displaced affects the buoyant force. - Buoyant force exerted by liquid does not depend upon its volume.
So, the buoyant force will not increase on increasing the volume of the liquid. - Hydrometer and lactometer are based on Archimedes’ principle. Submarines are also designed according to Archimedes’ principle.
Answer 21.
Vertebrata is divided into two superclasses, viz. Pisces and Tetrapoda. Animals of Pisces class have streamlined body with fins and tails to assist in swimming. Animals of Tetrapoda class have four limbs for locomotion.
Tetrapoda is further classified into following classes:
- Amphibia: Are adapted to live in water and on land. Can breathe oxygen through skin when under water.
- Reptilia: These are crawling animals. Skin is hard to withstand extreme temperatures.
- Aves: Forelimbs are modified into wings to assist in flying. Beaks are present. Body is covered with feathers.
- Mammalia: Mammary glands present to nurture young ones. Skin is covered with hair. Most of the animals are viviparous.
OR
SECTION-B
Answer 22.
The reaction produced gaseous product, CO2 which escaped into the atmosphere.
Answer 23.
Neuron has three parts:
- Cell body or cyton
- Dendrite
- Axon
Answer 24.
- Plant root cells absorb water by osmosis.
- The unicellular organisms like amoeba takes in the required materials by osmosis.
Answer 25.
Mass of solute = 40 g
Mass of solvent = 280 g
Mass of solution = Mass of solute + Mass of solvent.
= 40 g + 280 g
= 320 g
Mass percentage of solution = \(\frac { Massofsolute }{ Massofsolution }\) × 100
= \(\frac { 40 }{ 320 }\) × 100 = 12.5%
Answer 26.
The spring balance should be held only at the hook on the top end, never hold the spring balance from its sides.
Answer 27.
Boron + Oxygen → Compound of Boron and Oxygen
0.096 g + 0.144 g → 0.240 g
This can be accounted on the basis of law of conservation of mass.
We hope the CBSE Sample Papers for Class 9 Science Paper 2 help you. If you have any query regarding CBSE Sample Papers for Class 9 Science Paper 2, drop a comment below and we will get back to you at the earliest.