CBSE Sample Papers for Class 9 Science Paper 3 are part of CBSE Sample Papers for Class 9 Science. Here we have given CBSE Sample Papers for Class 9 Science Paper 3.
CBSE Sample Papers for Class 9 Science Paper 3
| Board | CBSE |
| Class | IX |
| Subject | Science |
| Sample Paper Set | Paper 3 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 9 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme as prescribed by the CBSE is given here. Paper 3 of Solved CBSE Sample Paper for Class 9 Science is given below with free PDF download solutions.
Time Allowed : 3 Hours
Max. Marks: 80
General Instructions
- The question paper comprises of two Sections, A and B. You are to attempt both the sections.
- All questions are compulsory. However an internal choice will be provided in two questions of 3 marks each and one question of five marks.
- All questions of Section A and all questions of Section B are to be attempted separately.
- Question numbers 1 to 2 in Section A are one-mark questions. These are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section A are two-marks questions. These are to be answered in about 30 words each.
- Question numbers 6 to 15 in Section A are three-marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section A are five-marks questions. These are to be answered in about 70 words each.
- Question numbers 22 to 27 in Section B are two-marks questions based on practical skills. These are to be answered in brief
Questions
SECTION-A
Question 1.
What is the charge and mass of proton?
Question 2.
What is haemocoel? Which group of animals have haemocoel?
Question 3.
The number of electrons in the outermost shell of chlorine is 7. What is its valency and why?
Question 4.
How will you differentiate between a primitive organism from the advanced organisms?
Question 5.
Explain why feather falls slowly than a coin under gravity through air.
Question 6.
State one feature that is similar and one feature that is dissimilar with respect to mitochondria and plastids.
Question 7.
What is meant by the term mole? Calculate the number of moles in
(a) 3.011 × 1023 atoms of C
(b) 32 g of oxygen gas
[NA = 6.022 × 1023 mol-1, At. Mass of O = 16 u and C = 12 u]
Question 8.
State the distinguishing characteristics of the division Thallophyta.
OR
State three ways in which phloem is different from Xylem.
Question 9.
List the names of three diseases caused by virus stating their mode of communication in each mode.
OR
- Why is making of anti-viral medicines harder than anti-bacterial medicines?
- How can we prevent the exposure to infectious microbes?
Question 10.
Define pressure and its S.I. Unit. The dimensions of a metallic cuboid are 30 cm × 20 cm × 15 cm and its mass is 30 kg. If the acceleration due to gravity be 10 m/sec2, Calculate the pressure exerted by the cuboid, when it is resting on the face having sides 20cm × 15 cm on a table.
Question 11.
State the law of conservation of momentum. Why a person is hit harder when he falls on a hard floor than when he falls on a heap of sand from the same height?
A bullet of mass 20 g is fired horizontally with a velocity 100 m/s from a pistol of mass 1.5 kg. Calculate the recoil velocity of the pistol.
Question 12.
Give an example in each case where work done by a force is:
- Zero
- Positive
- Negative
Question 13.
A goldsmith measured the purity of gold by using a special measuring device. He told the customer that there was impurity present in gold ornament that he wanted to buy and it was not 22 carats but 18 carats jewellery.
- How can we find the purity of gold?
- What is the unit of relative density?
- What value of goldsmith is reflected in this act?
Question 14.
Silver nitrate solution was mixed with 5 g of sodium chloride solution to verify the law of conservation of mass. 8.1 gram of silver chloride was formed and sodium nitrate formed was equal to half of the amount of silver nitrate solution used. What is the amount of AgNO3 used and NaNO3 formed.
Question 15.
- Why are the roofs and walls of an auditorium hall generally covered with sound absorbent materials?
- The sound of ringing bell inside a vacuum chamber can’t be heard. Why?
Question 16.
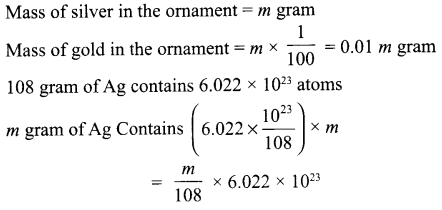
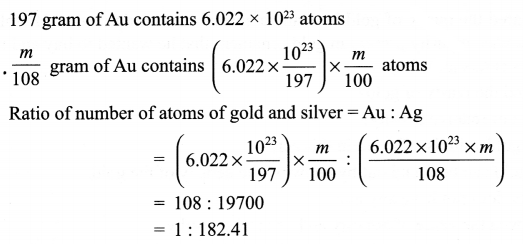
A silver ornament of mass ‘m’ grams is polished with gold equivalent to 1% of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Question 17.
Explain the structure of the human ear with the help of a diagram.
Question 18.
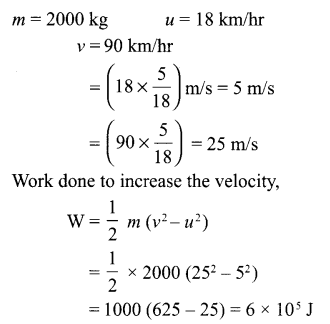
What do you mean by work? Give an example of negative work done? What is the work done to increase the velocity of a car from 18 km/hr to 90 km/hr, if the mass of the car is 2000 kg.
Question 19.
- State two factors on which the magnitude of buoyant force acting on a body immersed in a fluid depends.
- Will the buoyant force exerted by a liquid increase, if its volume is increased?
- Name some devices based on the Archimedes principle.
Question 20.
Continuous addition of fertilisers to the soil destroys its fertility. Long term fertilisers use is substituted with the various cropping pattern to maintain the fertility of the soil. Discuss few cropping patterns, their techniques and benefits.
OR
- Define vaccine. Name two vaccines given to children.
- What is antibiotic penicillin? Explain its functioning.
Question 21.
- List four main processes involved in the water cycle.
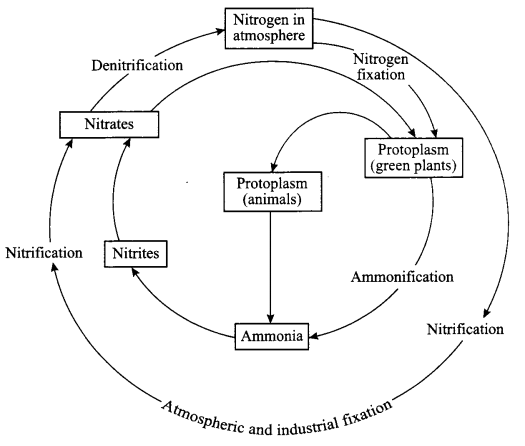
- Give a diagrammatic representation of Nitrogen cycle in nature.
SECTION-B
Question 22.
The length, breadth and height of a cuboid of mass m kg are 5 cm, 3 cm and 2 cm respectively. Calculate the surface area of the surface where the pressure on the floor will be maximum.
Question 23.
Write the difference between male and female cone of Pinus?
Question 24.
In a chemical reaction, sodium carbonate (5.3 g) reacts with and ethanoic acid (6 g) forms (8.2 g) sodium ethanoate, (2.2 g) carbon dioxide and (0.9 g) water.
Does the product formed follow the law of conservation of mass.
Question 25.
In a plant, name two features which you would examine to categorise it into a monocot or a dicot plant.
Question 26.
How does a cockroach adapt itself to a wide range of habitats?
Question 27.
What are the different adulterants commonly found in foods?
Answers
SECTION-A
Answer 1.
Proton has positive charge. Its mass is 1.675 × 10-27 kg.
Answer 2.
Haemocoel is a pseudocoel with blood. It is found in arthropods and molluscs.
Answer 3.
Its valency is one because it can gain one electron to become stable like its nearest noble gases argon.
Answer 4.
The group of organisms which have ancient body designs and have not changed much are called “primitive” organisms. They are different from the advanced organisms as advanced organisms are group of organisms which have acquired their particular body designs recently.
Answer 5.
Air resistance depends on the surface area which is more in case of feather.
Answer 6.
Similar feature: Both have their own DNA and ribosome to synthesise their own proteins.
Dissimilar feature: Mitochondria is the site of cellular respiration and release energy, while plastids with chlorophyll are centre for photosynthesis and store energy.
Answer 7.
Mole is defined as the counting unit at atomic & molecular level and is equal to 6.022 × 1023 atoms which is the number of atoms 12 g of 12C isotope.
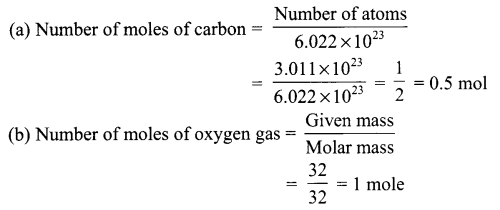
Answer 8.
The characteristic features of the division Thallophyta are:
- The plant body consists of individual thallus.
- The sex organs are single celled.
- After fertilisation, no embryo is formed.
OR
- Phloem allows the movement of materials in both the directions and Xylem allows the movement in only one direction(upwards).
- Phloem transports food from leaves to all parts of the plant body and Xylem transports water and minerals from roots to all the parts of the plant body.
- Phloem does not use suction pull force like xylem and it does not many dead cells as xylem has.
Answer 9.
- Jaundice – Contaminated food and water.
- Rabies – Saliva of infected animal.
- AIDS – Through sexual contact, blood transfusion, reusing the contaminated needle, from infected mother to the foetus.
OR
1. Antibiotics block the bacterial process that builds the cell wall in bacteria. As a result, the growing bacteria become unable to make cell wall and dies away easily. Viruses do not have any biochemical mechanism of their own. They enter our cells and use our machinery for their life processes. Therefore making of anti-viral medicine is difficult than anti-bacterial medicine.
2. We can prevent exposure to airborne microbes by providing living conditions that are not overcrowded. Exposure to waterborne microbes can be prevented by providing safe . drinking water. This can be done by proper scientific way of treating the water to kill any microbial contamination. Exposure to vector-borne microbes can be prevented by living in a clean environment.
Answer 10.
Pressure is defined as thrust per unit area (or force acting per unit area). Its SI unit is Pascal, (Pa)
m = 30 kg
A = 20 cm × 15 cm = 300 cm2
= 0.03 m2
g = 10 m/s2
p = \(\frac { mg }{ V }\) = \(\frac { 30\times 10 }{ 0.03 }\)
= 104 Pa
Answer 11.
Law of conservation of momentum: The sum of the momenta of the two objects before collision is equal to the sun of the momenta after collision, provided there is no external unbalanced force acting on them.
When a person falls on the hard floor, he is brought to rest in a very short interval of time, so a greater force come into play.
On the other hand when he falls on a heap of sand, he is brought to rest in a longer time, so lesser force come into play/explanation in terms of momentum.
Total momentum before firing (pistol & bullet) = 0
Total momentum after firing (of pistol & bullet) is
= 0.02 kg × ( 100 m s-1) + 1.5 kg × v ms-1
= (2 + 1.5 v) kg ms-1
Total momenta after firing = total momenta before firing
2 + 1.5 v = 0
1.5 v = -2
∴ v = 1.33 m/s
Answer 12.
- work done by gravity on a rolling ball.
- hitting a stationary ball.
- work done by a friction of a rolling bail.
Answer 13.
- The purity of gold can be found out by knowing the density of the gold.
- Relative density does not have any unit.
- Goldsmith showed the value of honesty and he is trustworthy.
Answer 14.
Let the mass of AgNO3 used be x.
Then, mass of NaNO3 formed will be \(\frac { x }{ 2 }\)
Mass of reactants = Mass of products
x + 5 = 8.1 + \(\frac { x }{ 2 }\)
\(\frac { x }{ 2 }\) = 3.1
x = 6.10 g
Mass of AgNO3 Used = 6.10 g
Mass of NaNO3 formed = \(\frac { 6.10 }{ 2 }\) = 3.05 g
Answer 15.
- If absorbent materials are absent then there will be multiple echoes, due to which sound cannot be heard clearly.
- Sound waves are mechanical waves and cannot travel through vaccum.
Answer 16.
Answer 17.
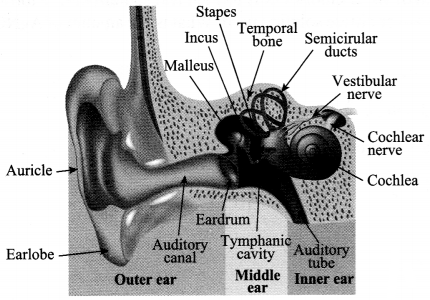
(a) Outer Ear: Pinna, auditory canal and tymphanic membrane.
- Pinna – It collects the sound from the surroundings.
- Auditory Canal – The sound waves collected pass through this canal.
- Tymphanic Membrane – It is a thin membrane which receives the vibrations of sound. A compression reaches the ear drum, the pressure on the outside of the membrane increases and pushes the ear drum inwards, and moves out when the rarefaction reaches the ear drum.
(b) Middle Ear: Consists of three small bones called hammer, anvil and stirrup. The vibrations are received by these three bones and the strength of vibrations is increased i.e., the sound is amplified and passed to inner ear.
(c) Inner Ear: It consist of cochlea and auditory nerve. Cochlea receives the amplified vibrations and convert them into electrical signals. These electrical signals are sent to the brain via the auditory nerve and brain interprets the signals as sound.
Answer 18.
Work is said to be done when the force applied on an object moves it in its own direction.
Example of negative work done – When a body is sliding on a surface, work done by force of friction is negative.
Answer 19.
- Buoyant force = Weight of liquid displaced
= (Volume of the liquid displaced) × (Density of liquid) × (Acceleration due to gravity)
So, density of liquid and volume of the liquid displaced affects the buoyant force. - Buoyant force exerted by liquid does not depend upon its volume.
So, the buoyant force will not increase on increasing the volume of the liquid. - Hydrometer and lactometer are based on Archimedes’ principle. Submarines are also designed according to Archimedes’ principle.
Answer 20.
Three different cropping patterns, namely mixed cropping, inter-cropping and crop-rotation are generally practised.
1. Mixed cropping allows two or more crops to be owed simultaneously on the same piece of land. Wheat and gram, wheat and mustard, groundnut and sunflower etc. are some common examples of crops grown through mixed cropping. Crops are chosen in such a way that they require different amounts of minerals.
2. Inter-cropping allows farmers to grow two or more crops simultaneously in the same field in a definite pattern. For example, cauliflower and chilli plants are grown together in alternating rows on a single field. To ensure that maximum utilisation of the nutrients applied, crops are selected in such a way that their nutrient requirements are different. Other examples of crops grown through inter-cropping include soyabean and maize, finger millet (bajra) and cow-pea (lobia) etc.
3. Crop-rotation is the practice of growing two or more varieties of crops in the same field in sequential seasons. A common example of crop-rotation is to cultivate maize followed . by soyabean. This system helps in protecting crops from pests and diseases. The crops selected, vary in nutrient requirements. This ensures complete and uniform utilisation of the nutrients applied.
OR
- Vaccine is a chemical/drug given in advance to a body to give immunity against certain diseases.
Two vaccines given to children are:- BCG – for tuberculosis prevention
- Polio drops – for polio prevention
- Antibiotic penicillin is a vaccine which blocks the bacterial processes that build the cell wall when the bacteria infects a living person. Due to this drug, the bacteria is unable to make a protective cell wall and it dies away easily. It is used to cure the diseases and infections caused by bacteria.
Answer 21.
1. Evaporation – Condensation – Transpiration – Precipitation
2.
SECTION-B
Answer 22.
Surface having dimensions 3 cm and 2 cm, respectively, having surface area = (3 × 2) cm2 has more pressure,
∵ Pressure is inversely proportional to area. If area is less, pressure is more.
Answer 23.
- Female cones are large and woody.
- Male cones are smaller and tender.
Answer 24.
Sodium carbonate + Ethanoic acid → Sodium ethanoate + Carbon Dioxide + Water.

∵ It follows the law of conservation of mass.
Answer 25.
In any given plant to classify it as monocot or dicot, we need to see the venation in leaves, reticulated net like pattern refers to dicot plant and parallel venation refers to monocot plant.
Answer 26.
Cockraoch has a hard exoskeleton that serves many functions, including protection from water, loss and physical injury and internally required for muscle attachment, which results in tremendous leverage and strength. It also undergoes hibernation during unfavorable conditions.
Answer 27.
The commonly used adulterants in food are:
- Black pepper – dry seeds of papaya
- Honey – Jaggery
- Mustard seeds – argemone seeds
- Red chilli powder – red brick powder
We hope the CBSE Sample Papers for Class 9 Science Paper 3 help you. If you have any query regarding CBSE Sample Papers for Class 9 Science Paper 3, drop a comment below and we will get back to you at the earliest.