CBSE Sample Papers for Class 9 Science Paper 5 are part of CBSE Sample Papers for Class 9 Science. Here we have given CBSE Sample Papers for Class 9 Science Paper 5.
CBSE Sample Papers for Class 9 Science Paper 5
| Board | CBSE |
| Class | IX |
| Subject | Science |
| Sample Paper Set | Paper 5 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 9 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme as prescribed by the CBSE is given here. Paper 5 of Solved CBSE Sample Paper for Class 9 Science is given below with free PDF download solutions.
Time Allowed : 3 Hours
Max. Marks: 80
General Instructions
- The question paper comprises of two Sections, A and B. You are to attempt both the sections.
- All questions are compulsory. However an internal choice will be provided in two questions of 3 marks each and one question of five marks.
- All questions of Section A and all questions of Section B are to be attempted separately.
- Question numbers 1 to 2 in Section A are one-mark questions. These are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section A are two-marks questions. These are to be answered in about 30 words each.
- Question numbers 6 to 15 in Section A are three-marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section A are five-marks questions. These are to be answered in about 70 words each.
- Question numbers 22 to 27 in Section B are two-marks questions based on practical skills. These are to be answered in brief
Questions
SECTION-A
Question 1.
Why is evaporation called a surface phenomenon?
Question 2.
Kingdom Fungi have cell wall but it is not classified under kingdom Plantae. Give reason.
Question 3.
What is chromatography? Give its one application.
Question 4.
What are isotopes and isobars?
Question 5.
What is the commercial unit of energy? Define it.
Question 6.
Name the Italian bee variety commonly used for commercial honey production. What are the qualities of this bee which makes it most suitable?
Question 7.
Which separation technique is used to separate the following:
- Cream from milk
- Oil from ester
- Camphor from mixture containing camphor and sodium chloride
Question 8.
Explain why:
- Lysosomes are known as suicidal bags.
- Mitochondria are known as powerhouse of the cell
OR
What are ribososmes? Where are they located in the cell? What is their function?
Question 9.
Why is excess use of fertilisers harmful for environment?
Question 10.
If bromine atom is available in the form of two isotopes say \(_{ 35 }^{ 79 }{ Br }\) (49.7%) and \(_{ 35 }^{ 81 }{ Br }\) (50.3%), calculate the average atomic mass of bromine atom.
Question 11.
What is AIDS? How does a person get affected with HIV?
Question 12.
A boy possess potential energy of 460 J whose mass is 20 kg and is raised to a certain height.
What is the height. [Take g = 10m/s2]
OR
A car accelerates uniformly from 20 km/h to 35 km/h in 5 s. Calculate
(i) the acceleration and
(ii) the distance covered by the car in that time?
Question 13.
- Under which category of mixtures will you classify alloys and why?
- A solution is always a liquid. Comment.
- Can a solution be heterogeneous? Justify.
Question 14.
Establish the relationship between speed, wavelength and frequency of sound waves.
Question 15.
What is acid-rain? Give its harmful effects.
Question 16.
Explain Rutherford’s a-particle scattering experiment and give its observation and conclusion drawn from the experiment.
Question 17.
Represent graphically:
(a) Two sound waves having same amplitude but different frequencies.
(b) Two sound waves having different amplitudes and also different wavelengths.
(c) Two sound waves having same frequency but different amplitude.
(d) Also write the characteristic of sound obtained.
Question 18.
Explain different types of fisheries.
Question 19.
State Archimedes principle. State laws of floatation. Explain why iron nail sinks but iron boat floats. Why is it easier to swim in sea water than in river water?
Question 20.
- How carbon exists in nature?
- Explain the carbon-cycle in nature.
OR
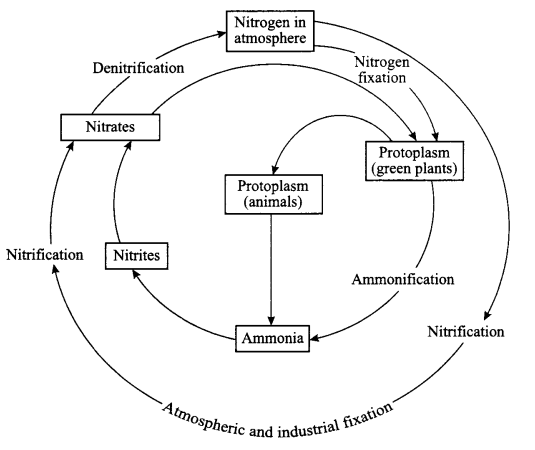
Explain nitrogen-cycle in nature and define all the terms involved in it.
Question 21.
Explain with examples:
- Atomic number
- Mass number
- Isotopes
- Isobars
Give any two uses of isotopes.
SECTION-B
Question 22.
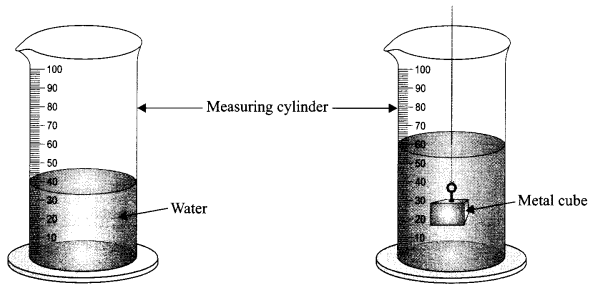
The water level in a measuring cylinder, before and after immersing a metal cube in it, is shown in the figure. Calculate the volume of the metal cube.
Question 23.
What would happen when zinc granules are added to dilute sulphuric acid in a test tube? Record any two observations.
Question 24.
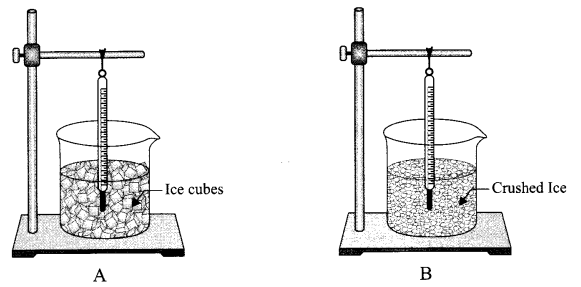
Which experimental set-up is correct for determining the melting point of ice? Why?
Question 25.
You can bite fruits like guava, grapes, banana etc., but not a piece of wood. Why?
Question 26.
Why does brass react with dilute hydrochloric acid and is corroded in rainy season to form CuCO3 . CU(OH)2?
Question 27.
State the life span of each stage of mosquito.
Answers
SECTION-A
Answer 1.
The water molecules at the surface gains heat energy, required to convert liquid water into vapours, hence it is called a surface phenomenon.
Answer 2.
Fungi does not have chloroplast to prepare its own food.
Answer 3.
It is a separating technique of two or more coloured mixtures or colourless mixtures. It can also be used to detect the impurities present in coloured food items.
Answer 4.
- Isotopes: Atoms of the same element with same atomic number but different mass number.
- Isobars: Atoms of diferrent elements with same mass number but different atomic number.
Answer 5.
k W h. When 1000 watt is used for one hour it is called 1 kW h.
Answer 6.
Apis Mellifera is commonly used bee.
The bee is stingless, produces more honey and lives at a single place for a long time.
Answer 7.
- Centrifugation
- Distillation
- Sublimation
Answer 8.
- Lysosomes helps to keep the cell clean by digesting out foreign waste materials and worn out cells (or cell organelles) with the help of powerful digestive enzymes that it contains. It can worn out the entire cell & burst out and thus known as suicidal bags of the cell.
- Mitochondria stores energy in the form of ATP molecules and provides the energy when needed by the cell. Hence, it is called the powerhouse of the cell.
OR
Ribosomes are spherical organelles present in the cell which are either freely distributed in the cytoplasm or may be attached to the endoplasmic reticulum. It consists of ribosomal RNA (Ribonucleic acid) and proteins.
Functions of Ribosomes: It helps in the synthesis of proteins.
Answer 9.
It changes the nature of the soil.
It lowers the water holding capacity of soil.
The chemical enters the food chain, as it is an inorganic chemical and not used by animals, so it gets accumulated in the top most consumers leading to biological magnification and can cause various harmful diseases like cancer.
Answer 10.
The average atomic mass of bromine atom
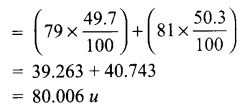
Answer 11.
AIDS means Acquired Immuno Deficiency Syndrome, which is caused due to HIV- human immuno deficiency virus. This virus reduces the immunity of the human body. Therefore if any pathogen enters the body of a person and causes any disease then it can kill the person. The virus is transmitted from an infected person to person by any of the following ways:
- Blood transfusion
- From mother (infected) to the baby in the womb
- From the infected mother’s milk to the lactating baby
- By sexual contact
- Sharing Reusing a needle used by an infected person
Answer 12.
P.E. = 460 J, m = 20 kg, g = 10 m/s2
h= ?
P.E. = mgh
460 = 20 × 10 × h
h = \(\frac { 460 }{ 20\times 10 } \)
h = 2.3 m
The height at which the object is raised from the ground is 2.3 m.
OR

Answer 13.
- Alloys are homogeneous mixtures as its constituents cannot be easily distinguished and have a uniform composition throughout.
- A solution is obtained when a solute (solid/liquid or gas) dissolves in a solvent. Generally, the solvent is a liquid, such solutions are called liquid solutions. However, it is not necessary that a solution is always a liquid.
- No, a solution cannot be heterogeneous as formation of a solution involves dissolving process, which always results in a homogeneous mixture.
Answer 14.
Speed of sound waves → The distance travelled by a sound wave or a point on a wave (compression or rarefaction) per unit time.
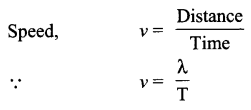
Where, X is wavelength of the sound wave and it is equal to the distance travelled by the sound wave in one time period (T) of the wave.
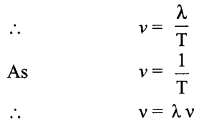
Speed = Wavelength × Frequency
Answer 15.
The harmful gases released due to combustion of fossil fuels are SO2, NO2, CO2, these gases remain suspended in the air. When it rains the rain water mixes with these gases to form sulphuric acid, nitrous acid, carbonic acid and comes down on the surface of the earth in the form of acid-rain.
Harmful effects of acid-rain:
- It corrodes statues, monuments of marble, building etc.
- It makes the soil acidic.
- It damages crops, plants.
Answer 16.
Rutherford’s α-particle scattering experiment:
Fast moving α-particles were made to fall on a thin gold foil. Particles have + 2 charge, 4 u mass and considerable amount of energy when hits the gold foil, following observations are made:
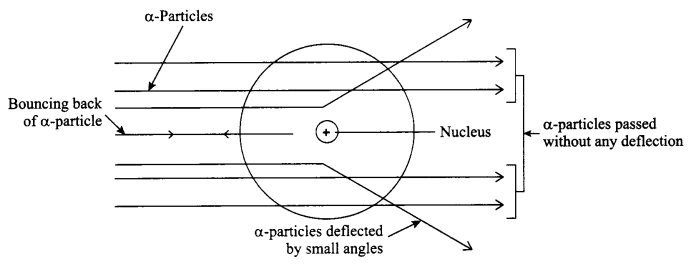
- Most of the α-particles passed straight through the foil without any deflection.
- Some of the α-particles were deflected by small angles through the foil.
- One out of every 20,000 particles were rebounded.
Conclusion:
- Most of the space inside the atom is empty.
- Mass of the atom is concentrated in the centre which is positively charged in a small volume within the atom.
- The positively charged centre of atom is called the nucleaus of the atom.
Answer 17.
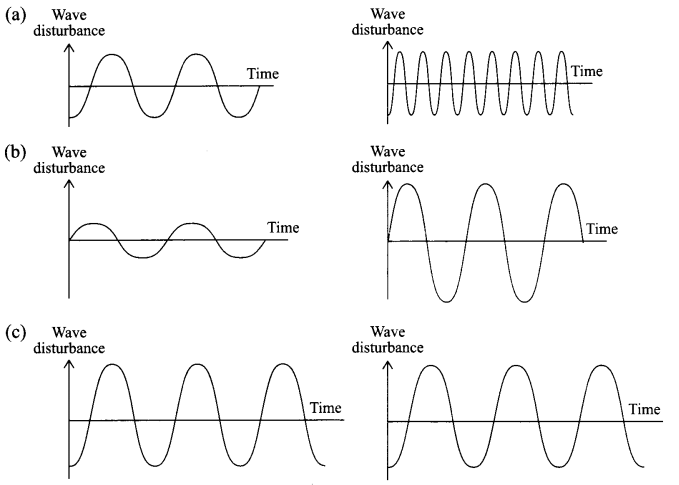
(d)
- Soft Sound
- Louder Sound
Answer 18.
The different types of fisheries are as follow:
- Marine fisheries: Marine fishes are caught using fishing nets. Large schools of fishes are located by satellites. Some are farmed in sea water.
- Mariculture: Marine fishes are cultured in sea water. This culture of fisheries is called mariculture.
- Inland fisheries: The fisheries done in fresh water resources like canals, ponds, reservoirs and rivers is called inland fisheries
- Capture fishing: It is done in sea water, estuaries and lagoons.
- Aquaculture: Culture of fishes done in different water bodies is called aquaculture.
Answer 19.
Archimedes Principle: When a body is immersed in a fluid (fully or partially), it experiences an upward force which is equal to the weight of the fluid displaced by it.
Law of Floatation:
- A freely floating body in a fluid displaces the weight of the fluid equivalent to its own weight.
- For a freely floating body, the centre of gravity of the floating body and the centre of gravity of the fluid in which it floats are in the same vertical line.
An iron nail sinks due to its high density and less buyoant force acting on the nail, while the iron boat floats because of more buyoant force acting due to increase in surface area.
It is easier to swim in sea water rather than river because the surface of sea water is more dense than river water and its is easier to float on a dense surface.
Answer 20.
1. Carbon exist in two different forms in nature-free and combined form.
- Free form: It occurs in the elemental form as diamond and graphite.
- Combined form: It occurs as carbon-dioxide, carbonate and hydrogen carbonate salts in various minerals. It is also present in proteins, carbohydrates, fats, nucleic acids and vitamins.
2. Carbon cycle:
- Carbon-dioxide present in the nature is used by the plants during photosynthesis to form glucose and carbohydrates.
- CO2 dissolves in water to form carbonates, which forms limestone.
- Plants contain carbon in the form of glucose, carbohydrates, which are eaten up by the other animals.
- Animals form petroleum when submerged under the Earth and plants form coal. But if get decomposed after dying they release carbon back to the atmosphere by decomposition (dead) and respiration (living).
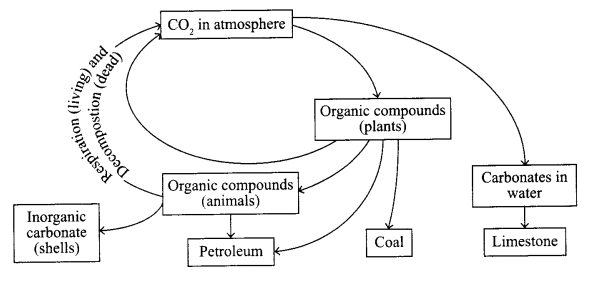
OR
Terms involved in nitrogen-cycle are
- Nitrogen fixation: Plants cannot use free nitrogen present in the air. This nitrogen molecule is converted into nitrates and nitrites which can be taken up and used to make the required molecule. This is called nitrogen fixation, which can be done by the bacteria that live in the root nodules of leguminous plants.
- Nitrification: By physical process i.e. during lightning, the high temperature and pressure created in the air converts nitrogen into oxides of nitrogen which dissolve in water and came down along with the rain. This is called nitrification.
- Ammonification: The nitrogen compounds formed after nitrificaiton are taken by the plants to form proteins which are further converted into ammonia.
- Denitrification: The nitrates and nitrites of nitrogen are acted upon by some group of microbes, e.g. Pseudomonas bacteria, which converts these compounds into free nitrogen gas.
Nitrogen cycle:
- Free nitrogen from atmosphere is converted into nitrates by bacteria or by lightning.
- Nitrates mixes with soil and is absorbed by the plants to make proteins.
- The proteins in plants and animals are converted into amino acids and ammonia.
- Ammonia is converted into nitrates and then these nitrates and nitrites present in soil is acted upon by a group of bacteria called denitrifying bacteria. This process is called denitrification. Nitrates are converted into free nitrogen and is released back to the atmosphere.
Answer 21.
- Atomic number: The atomic number of an element is equal to the number of protons in the nucleus of its atom e.g., Oxygen has 6 protons hence atomic no. = 6. 1
- Mass number: The mass number of an atom is equal to the number of protons and neutrons in its nucleus.
Mass Number = Number of protons + Number of neutrons - Isotopes: Isotopes are the atoms of the same element which have different mass number but same atomic number, e.g., \(_{ 1 }^{ 1 }{ Br }\), \(_{ 1 }^{ 2 }{ Br }\), \(_{ 1 }^{ 3 }{ H }\)
- Isobars: Isobars are atoms having the same mass number but different atomic numbers. e.g; \(_{ 20 }^{ 40 }{ Ca }\), \(_{ 18 }^{ 40 }{ Ar }\)
Both calcium and argon have same mass number but different atomic number.
Two uses of isotopes are:
- An isotope of iodine is used in the treatment of goitre.
- An isotope of uranium is used as a fuel in nuclear reactors.
SECTION-B
Answer 22.
Initial volume of water = 40 cm3
Final volume of water after immersing the metal cube = 60 cm3
Volume of the metal cube = 60 – 40 = 20 cm3
Answer 23.
- The bubbles/effervescence will be seen in the test tube and zinc granules will keep on disappearing.
- The test tube will become warm.
Answer 24.
The set-up B is correct as the beaker contains crushed ice and the thermometer is in contact with the ice.
Answer 25.
The skin of fruits like apple, grapes guava is very soft and made up of soft tissues. On the other hand, the wood has many layers of dead cells which makes it hard.
Answer 26.
Brass is an alloy of copper and zinc. It is an homogeneous mixture. The combining elements retain their properties. Hence the copper in brass reacts with the carbonates in the air to form CuCO3 . CU(OH)2.
Answer 27.
| Stage | Name | Life-Span |
| I | Egg | 24-48 hours |
| II | Larva | 7-14 days |
| III | Pupa | 1 -4 days |
| IV | Adult | Male – around one week Female – around one month |
We hope the CBSE Sample Papers for Class 9 Science Paper 5 help you. If you have any query regarding CBSE Sample Papers for Class 9 Science Paper 5, drop a comment below and we will get back to you at the earliest.