Learninsta presents the core concepts of Microbiology with high-quality research papers and topical review articles.
Energy of Chemical Reaction
Light energy is trapped by phototrophs during photosynthesis, in which it is absorbed by bacteriochlorophyll and other pigments and converted to chemical energy for cellular work. The energy is required by the bacterium for synthesis of cell wall or membrane, synthesis of enzymes, cellular components, repair
mechanism, growth and reproduction.
Some change of energy occurs whenever bonds between atoms are formed or broken during chemical reactions. When a chemical bond is formed, energy is required. Such a chemical reaction which requires energy is called an endergonic reaction (energy is directed inward). When a bond is broken, energy is released. A chemical reaction that release energy is an exergonic reaction (energy is directed outward).
During chemical reaction energy is either released or absorbed and the quantum of energy liberated or taken up is useful energy and is referred to Free Energy Change (ΔG) of the reactions.
High Energy Phosphate
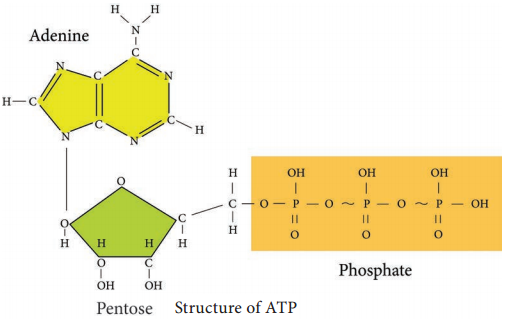
Adenosine Tri-Phosphate (ATP) is the principal energy carrying molecule of all cells and is indispensable to the life of the cell. It stores the energy released by some chemical reactions, and it provides the energy for reactions that require energy. ATP consists of an adenosine unit composed of adenine, ribose with three phosphate groups. In ATP and some other phosphorylated compounds, the outer two phosphate groups are joined by an anhydride bond.
Some of the other high energy nucleotides involved in biochemical processes are given in Table 4.1.
Table 4.1: High energy nucleotides involved in biosynthesis
|
Name of the Nucleotide |
Biosynthesis |
| Uridine triphosphate (UTP) | Polysaccharide |
| Cytidine triphosphate (CTP) | Lipid |
| Guanidine triphosphate (GTP) | Protein |
Nutrients are broken from highly reduced compounds to highly oxidized compounds within the cells. Much of the energy released during oxidation reduction reactions is trapped within the cell by the formation of ATP. A phosphate group is added to ADP with the input of energy to form ATP.
ATP + H2O → ADP + pi(ΔG° = – 7.3 K cal/mol)
ATP + H2O → AMP + ppi(ΔG° = – 10.9 K cal/mol)
ATP is ideally suited for its role as an energy currency. It is formed in energy trapping and energy generating processes such as photosynthesis, fermentation, and aerobic respiration. In bacterial and archeal cells, most of the ATP is formed on the cell membrane, while in eukaryotes the reactions occur primarily in the
mitochondria (Figure 4.2).
Oxidation – Reduction Reactions
Oxidation is the removal of electrons (e–) from an atom or molecule and is often an energy producing reaction. Reduction of a substrate refers to its gain or addition of one or more electrons to an atom or molecule. Oxidations and reduction are always coupled. In other words, each time one substance is oxidized, another is simultaneously reduced.
F2 + 2e– → 2F–
H2 + 2e– → 2H+ + 2e–
NAD+ + 2H+ + 2e– ⇄ NADH + H+.