NCERT Solutions for Class 12 Chemistry Chapter 5 are the practice guide for all the students. It contains solved questions provided by the subject matter experts. The solutions are accurate and the answers can be written in the exams.
NCERT Solutions are provided for reference in various boards (CBSE, UP board, Gujarat board, MP board). These help the students practice well before the examination and know their shortcomings. NCERT Solutions not only help the students score well in the board exams but also help them get through competitive exams.
| Board | CBSE |
| Textbook | NCERT |
| Class | Class 12 |
| Subject | Chemistry |
| Chapter | Chapter 5 |
| Chapter Name | Surface Chemistry |
| Number of Questions Solved | 35 |
| Category | NCERT Solutions |
NCERT Solutions for Class 12 Chemistry Chapter 5 Surface Chemistry
Class 12 Chemistry Chapter 5 Surface Chemistry is an important chapter and explains the types of chemical reactions, its pocesses and mechanisms that take place at the surface of the material. The chapter also contains the details on various important concepts such as Tyndall effect, Brownian motion, and catalyst reactions. Notes on various topics like colloids, emulsions, electrodialysis, enzyme reactions, electrophoresis, etc. are also provided here for reference.
NCERT IN-TEXT QUESTIONS
Question 1.
Why are substances like platinum and palladium often used for carrying out the electrolysis of aqueous solutions ?
Answer:
The metals like platinum and palladium are used as inert electrodes for carrying out the process of electrolysis because these are not attacked by the ions involved in the process.
Question 2.
Why does physisorption decrease with increase in temperature ?
Answer:
Physisorption or physical adsorption of a gas on the surface of a solid is exothermic in nature.
![]()
When temperature is increased, the equilibrium gets shifted in the backward direction to neutralise the effect of increase in temperature. Consequently, physisorption decreases with the increase in temperature.
Question 3.
Why are powdered substances more effective . adsorbents than their crystalline forms?
Answer:
Powdered substances have greater surface area as compared to their crystalline forms. Greater the surface area, greater is the adsorption.
Question 4.
Why is it necessary to remove CO when ammonia is obtained by Haber’s process ?
Answer:
Carbon monoxide (CO) acts as a poison for the catalyst iron as well as promoter molybdenum which are used in the Haber’s process. Moreover, it is likely to combine with iron to form iron carbonyl Fe(CO)5. Therefore, it is necessary to remove it from the reaction mixture by suitable means.
Question 5.
Why is ester hydrolysis slow in the beginning and becomes fast after sometime ?
Answer:
In ester hydrolysis, an acid and alcohol are formed as the products. For example,
![]()
Acid will release H+ ions in solution which act as catalyst (auto-catalysis) for the reaction. That is why, the hydrolysis is slow in the beginning and becomes faster later on.
Question 6.
What is the role of desorption in the process of catalysis?
Answer:
Desorption makes the surface of the solid- catalyst-free for fresh adsorption of the reactants on the surface.
Question 7.
What modification can you suggest for Hardy-Schulze Law?
Answer:
According to Hardy-Schulze Law, the ions carrying charge opposite to the charge on sol particles neutralise their charge and thus cause their coagulation or precipitation. The law takes into account the charge carried by the ion and not its size. Smaller the size of the ion more will be its polarising power. Thus, the law should be modified in terms of the polarising power of the flocculating ion or the ion causing the precipitation. The modified form of the law states that “Greater the polarising power of the flocculating ion added, greater is its power to cause precipitation.”
Question 8.
Why is it essential to wash the precipitate with water before estimating it quantitatively?
Answer:
Some amounts of the electrolyte are mixed to form the ppt. Some of these electrolytes remain adsorbed on the surface of the particles of the ppt. Hence, it is essential to wash the ppt with water to remove the sticking electrolytes (or any other impurities) before estimating it quantitatively.
NCERT EXERCISE
Question 1.
Distinguish between the meaning of terms adsorption and absorption. Give one example in each case.
Answer:
Differences between Adsorption and Absorption:
Adsorption:
- It is a process as a result of which one substance gets concentrated only on the surface of the other.
- The concentration of adsorbate on the surface of the adsorbent is different than in the bulk.
- It is a surface phenomenon.
- Example: Adsorption of water vapour on silica gel.
Absorption:
- It is a process as a result of which one substance gets uniformly distributed in the volume of the other.
- Concentration is uniform in the entire solid system.
- It is a bulk phenomenon.
- Example: Adsorption of water vapour by dry calcium chloride.
Question 2.
What is the difference between physisorption and chemisorption?
Answer:

Question 3.
Why is a finely divided substance more effective as an adsorbent ?
Answer:
With the increase in surface area of adsorbent adsorption increases. Thus, in the powdered state (finely divided substance) or in porous state surface area of metals is more. Therefore, adsorption is more in these states.
Question 4.
What are the factors which influence the adsorption of a gas on a solid ?
Answer:
There are various factors that affect the rate of adsorption of a gas on a solid surface.
- Nature of the gas: Easily liquefiable gases such as NH3, HCl, etc. are adsorbed to a great extent in comparison to gases such as H2, O2, etc. This is because Van der Waal’s forces are stronger in easily liquefiable gases.
- The surface area of the solid: The greater the surface area of the adsorbent, the greater is the adsorption of a gas on the solid surface.
- Effect of pressure: Adsorption is a reversible process and is accompanied by a decrease in pressure. Therefore adsorption increases with an increase in pressure.
- Effect of temperature: Adsorption is an exothermic process. Thus in accordance with Leehatelie’s principle, the magnitude of adsorption decreases with an increase in temperature.
Question 5.
What is adsorption isotherm ? Distinguish between Freundlich adsorption isotherm and Langmuir adsorption isotherm.
Answer:
Adsorption isotherm represents the variation of the amount of the gas adsorbed and the corresponding pressure at a certain temperature. The mathematical forms of the two adsorption isotherms are :

The main points of distinction in the two adsorption isotherms are:
- Freundlich Adsorption isotherm is applicable to all types of adsorption whereas Langmuir Adsorption isotherm is applicable mainly to chemical adsorption or chemisorption.
- Freundlich adsorption isotherm fails at high pressure of the gas whereas Langmuir Adsorption isotherm can be applied under all pressures.
Question 6.
What do you understand by activation of adsorbent? How is it achieved?
Answer:
By activating an absorbent, we tend to increase the adsorbing power of the adsorbent. Some ways to activate an adsorbent are:
- By increasing the surface area of the adsorbent. This can be done by breaking it into smaller pieces or powdering it.
- Some specific treatments can also lead to the activation of the adsorbent.
For example, wood charcoal is activated by heating it between 650K and 1330K in vacuum pr air. It expels all the gases absorbed or adsorbed and thus, creates a space for the adsorption of gases.
Question 7.
What role does adsorption play in heterogeneous catalysis?
Answer:
Heterogeneous catalysis is generally carried on the surface of the finely divided metals of the transition series. Due to the availability of large surface area, the reacting species get adsorbed on the surface either by physical adsorption or by chemisorption. The adsorbed species get opportunity to mutually combine to form the products which are released or desorbed from the surface so as to accommodate more reacting species.
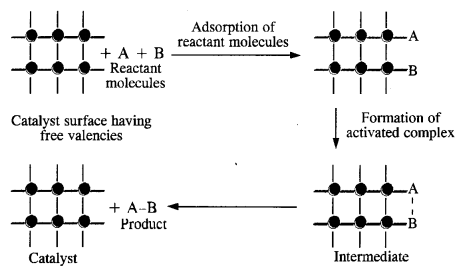
- Diffusion of the reactants on the surface of the catalyst.
- Some association between the catalyst surface and the reactants i. e., adsorption.
- The occurrence of the chemical reactions on the catalyst surface.
- Dissociation of the reaction products from the catalyst surface i.e., desorption.
- Diffusion of the products from the catalyst surface.
Question 8.
Give two chemical methods for the preparation of colloids.
Answer:
These are formed in two ways:
- Condensation methods
- Dispersion methods.
Condensation methods: The particles of the dispersed phase are very small in size. They have to be condensed suitably to be of colloidal size.
A colloidal solution of sulphur is obtained when H2S gas is bubbled through the solution of oxidising agent like bromine water, sulphur dioxide, dilute HNO3 etc.
Dispersion methods: In these methods, bigger particles of a substance (suspension) are broken into smaller particles of colloidal dimensions. The substance whose colloidal solution is to be prepared, is first ground to coarse particles. It is then mixed with the dispersion medium to get a suspension
Question 9.
How are colloidal solutions classified on the basis of physical states of the dispersed phase and dispersion medium?
Answer:
There are in all eight types of colloidal solutions.
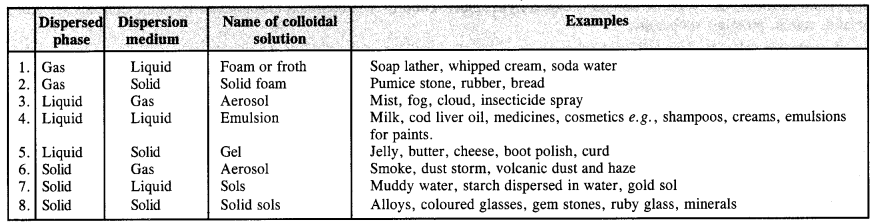
Question 10.
Discuss the effect of pressure and temperature on the adsorption of gases on solids.
Answer:
Effect of pressure:
Adsorption is a reversible process and is accompanied by a decrease in pressure. Therefore adsorption increases with an increase in pressure.
Effect of temperature:
Adsorption is an exothermic process. Thus, in accordance with Le-chatelier’s principle, the magnitude of adsorption decreases with an increase in temperature.
Question 11.
What are lyophilic and lyophobic sols? Give one example of each type. Why are hydrophobic sols easily coagulated?
Answer:
Lyophillic colloids (solvent loving) are those substances that directly pass into the colloidal state when brought- in contact with the solvent, e.g., proteins, starch, rubber, etc.
These sols are quite stable because of the strong attractive forces between the particles of disperse phase and the dispersion medium.
Lyophobic colloids (solvent hating) are those substances that do not form the colloidal sol readily when mixed with the dispersion medium.These sols are less stable than the lyophilic sols. Examples of lyophobic sols include sols of metals and their insoluble compounds like sulphides and hydroxides.
The stability of hydrophobic sol is only due to the presence of charge on the colloidal parties. If charge is removed, e.g., by addition of suitable electrolytes, the particles will come nearer to each other to form aggregate, i.e., they will coagulate and settle down. On the other hand, the stability of hydrophilic sol is due to charge as well as solvation of the colloidal particles. Thuf, for coagulation to occur easily both the mentioned factors have to be removed.
Question 12.
What is the difference between muitimolecular and macromolecular colloids ? Give one example of each. How are associated colloids different from these two types of colloids ? (C.B.S.E. 2008, 2009, 2010)
Answer:
Difference between multimolecular and macromolecular colloids
The main points of distinction are listed.
| Muitimolecular colloids | Macromolecular colloids |
|
1.The particle size is less than that of the colloidal range (< 103 pm) |
1. The particle size falls in the colloidal range (103 to 106 pm). |
Colloidal sol of sulphur (Sg) is an example of multimolecular colloid while colloidal sol of starch represents macromolecular colloid.
Associated colloids also called micelles, are generally electrolytes. They exist as ions at low concentrations. However, above a particular concentration called critical micellear concentration (CMC) and above a particular temperature called Kraft temperature (Tk), these get associated and exhibit colloidal behaviour. Soap is a common example of associated colloids.
Multimolecular colloids: In these colloids, the individual particles consist of an aggregate of atoms or small molecules with molecular size less than 103 pm. For example, gold sol consists of particles of various sizes having several atoms. Similarly, a sulphur sol consists of particles each having eight sulphur atoms (Sg). In these colloids, the particles are held by van der Waals’ forces.
Macromolecular colloids: In this type, the particles of the dispersed phase are sufficiently big in size (macro) to be of colloidal dimensions. These are normally polymers. A few naturally occurring macromolecules are starch, cellulose and proteins. The examples of artificial macromolecules are those of polythene, nylon, polystyrene, plastics etc.
Question 13.
What are enzymes? Write in brief the mechanism of enzyme catalysis.
Answer:
Enzymes are complex nitrogenous compounds which are produced by living plants and animals. In fact, these are proteins produced by living systems and catalyse certain biological reactions. These are, therefore, often known as bio-chemical catalysts and this phenomenon is known as bio-chemical catalysis.
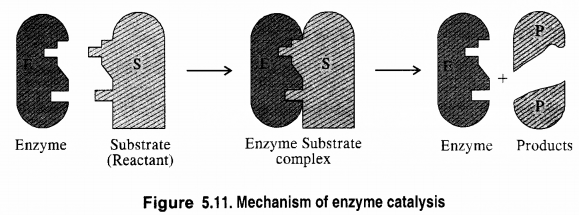
The rate of enzyme-catalyzed reaction which is initially of first-order changes to zero-order as the concentration of substrate species on the catalyst surface increases.
Two models have been proposed by bio-chemists to explain the mechanism of enzyme catalyzed reactions. These are briefly discussed.
Question 14.
How are colloids classified on the basis of :
(a) physical states of components
(b) nature of dispersion medium
(c) interaction between the dispersed phase and dispersion medium?
Answer:
(a) Based on physical states of components. Based on the physical states of components i.e., dispersed phase and dispersion medium, there are eight types of colloidal solutions.
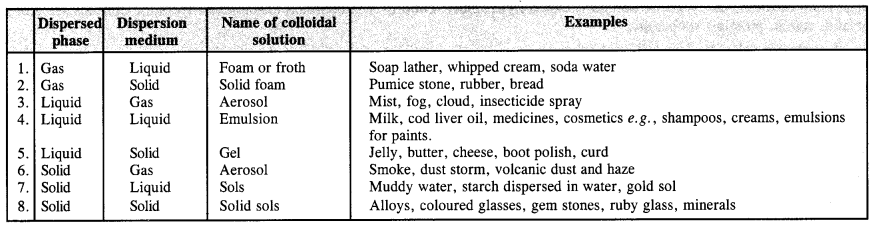
(b) Nature of dispersion medium. The dispersion medium can be either gas, liquid or solid. Based upon its nature, the colloids or colloidal solutions are of three types.
- Aerosols: Air or gases act as the dispersion medium
- Liquid sols: Liquids like water, alcohol or benzene act as the dispersion medium.
- Solid sols: Solid acts as the dispersion medium.
(c) Interaction between the dispersed phase and dispersion medium. Colloidal solutions are classified into two types. These are lyophilic and lyophobic sols.
(i) Lyophilic colloids: The colloidal solution in which the particles of the dispersed phase have a great affinity (or love) for the dispersion medium, are called lyophilic colloids. Such solutions are easily formed the moment the dispersed phase and the dispersion medium come in direct contact. e.g., sols of gum, gelatin, starch, etc.
(ii) Lyophobic colloids: The colloidal solutions in which the particles of the dispersed phase have no affinity or love, rather have hatred for the dispersion medium, are called lyophobic colloids. The solutions of metals like Ag and Au, hydroxides like Al(OH)3 and Fe(OH)3 and metal sulphides like As2S3 are examples of lyophobic colloids.
Question 15.
Explain what is observed
(i) when a beam of light is passed through a colloidal sol.
(ii) an electrolyte, NaCI is added to hydrated ferric oxide sol.
(iii) electric current is passed through a colloidal sol.
Answer:
(i) Scattering of light by colloidal particles takes place and the path of the light becomes visible (Tyndall effect).
(ii) The positively charged colloidal particles of Fe(OH)3 get coagulated by the oppositely charged Cl– ions provided by NaCl.
(iii) On passing electric current, the colloidal particles move towards the oppositely charged electrode where they lose their charge and get coagulated. This is the electrophoresis process.
Question 16.
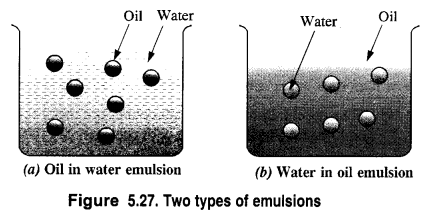
What are emulsions? What are their different types? Give an example of each type.
Answer:
(a) Oil-in-water emulsion (O/W type). In this case, the dispersed phase is oil while the dispersion medium is water. Milk is a common example in which liquid fats are dispersed in water. Similarly, if a few drops of nitrobenzene (oil) is added to water, an emulsion results. Vanishing cream is another example of this type.
(b) Water-in-oil emulsion (W/O type). In this type of emulsions, the dispersed phase is water while the dispersion medium is oil. Butter is an example of water in oil emulsion in which water is dispersed in oil. Cod liver oil and cold cream are the other examples of these emulsions.
Question 17.
What is demulsification? Name two demulsifiers.
Answer:
The process of separation of constituent liquids of an emulsion is called demulsification. Demulsification can be done by centrifuging or boiling.
Question 18.
The action of soap is due to demulsification and micelle formation. Comment.
Answer:
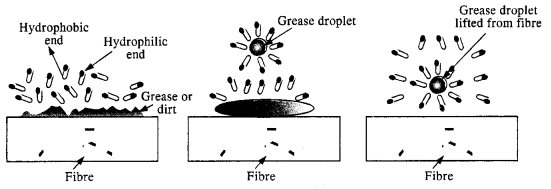
Soap is sodium or potassium salt of a higher fatty acid and may be represented as RCOO– Na+ (e.g., sodium stearate CH3(CH2)]16 COO– Na+, which is a major component of many bar soaps). When dissolved in water, it dissociates into RCOO– and Na+ ions. The RCOO– ions, however, consist of two parts – a long hydrocarbon chain R (also called non-polar tail’) which is hydrophobic (water-repelling), and a polar group COO– (also called polar ionichead’), which is hydrophilic (water loving).
The RCOO– ions are, therefore, present on the surface with their COO– groups in water and the hydrocarbon chains R staying away from it and remain at the surface. But at critical micelle concentration, the anions are pulled into the bulk of the solution and aggregate to form a spherical shape with their hydrocarbon chains pointing towards the centre of the sphere with COO– part remaining outward on the surface of the sphere. An aggregate thus formed is known as ‘ionic micelle’.
The cleansing action of soap is due to the fact that soap molecules form micelle around the oil droplet in such a way that the hydrophobic part of the stearate ions is in the oil droplet and the hydrophilic part projects out of the grease droplet like the bristles
Question 19.
Give four examples of heterogeneous catalysts.
Answer:
(i) The combination between nitrogen and hydrogen to form ammonia in the presence of finely divided iron acting as catalyst. This is known as Haber’s process.
![]()
(ii) Formation of sulphur trioxide by the oxidation of sulphur dioxide in the presence of platinum catalyst is the basis of the manufacture of sulphuric acid in Contact process.
![]()
(iii) Oxidation of ammonia into nitric oxide in the presence of platinum catalyst is employed for the commercial preparation of nitric acid in Ostwald process.
![]()
(iv) In the hydrogenation of vegetable oils (unsaturated in nature) resulting in solid fats (saturated in nature), hydrogen gas is passed through the oil in the presence of nickel catalyst at about 473 K.
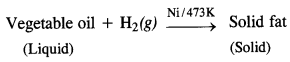
Question 20.
What do you mean by activity and selectivity of catalysts?
Answer:
(a) Activity: The activity of a catalyst depends upon the strength of chemisorption to a large extent. The reactants must get adsorbed reasonably strongly onto the catalyst to become active. But adsorption must not be so strong that they are immobilised. It is observed that maximum activity is shown by elements of groups 7 – 9 of the periodic table
2H2 + O2 \(\underrightarrow { Pt }\) 2H2O
(b) Selectivity: The selectivity of a catalyst is its ability to yield a particular product in the reaction e.g.,
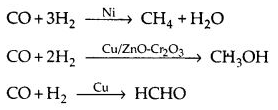
Thus, a selective catalyst can act as a catalyst in one reaction and may fail to catalyze another reaction.
Question 21.
Describe some features of catalysis by zeolites.
Answer:
- Zeolites are hydrated alumino-silicates. They have a three-dimensional network structure. They contain water molecules in their pores,
- Zeolites are heated to remove the water from hydration. The pores become vacant and zeolites are ready to act as catalysts.
- The size of the pores varies from 260 pm to 760 pm. This shows that only those molecules can be adsorbed in these pores whose size is small enough to enter these pores. Thus, zeolites a molecular sieve and the shape-selective catalysts.
Question 22.
What are shape-selective catalysts?
Answer:
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape-selective catalysis. Zeolites are good shape-selective catalysts because of their honeycomb-like structures. They are microporous aluminosilicates with a three-dimensional network of silicates in which some silicon atoms are replaced by aluminium atoms giving an Al-O-Si framework. The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as upon the pores and cavities of the zeolites. They are found in nature as well as synthesized for catalytic selectivity.
Question 23.
Explain the following terms:
(i) Electrophoresis
(ii) Coagulation
(iii) Dialysis
(iv) Tyndall effect.
Answer:
(i) Electrophoresis:
The movement of colloidal particles under the influence of an electric field is known as electrophoresis. Positively charged particles move to the cathode, while negatively charged particles move towards the anode. As the particles reach oppositely charged electrodes, they become neutral and get coagulated.
(ii) Coagulation:
The process of setting down colloidal particles i.e., conversion of a colloid into a precipitate is called coagulation.
(iii) Dialysis: The process of removing dissolved substances from a colloidal solution by the means of diffusion through a membrane is known as dialysis. This process is based on the principle that ions and small molecules can pass through animal membranes, unlike colloidal particles.
(iv) Tyndall effect:
When a beam of light is allowed to pass through a colloidal solution, it becomes visible like a column of light. This is known as the Tyndall effect. This phenomenon takes place as particles of colloidal dimension scatter light in all directions.
Question 24.
Give four uses of emulsions.
Answer:
Four uses of emulsions:
- the cleansing action of soaps is based on the formation of emulsions
- digestion of fats in the intestines takes place by the process of emulsification.
- Antiseptics and disinfectants when added to water form emulsions.
- The process of emulsification is used to make medicines.
Question 25.
What are micelles? Give an example of the micelles system.
Answer:
Micelles are substances that behave as normal strong electrolytes at low concentration but at high concentrations behave as colloids due to the formation of aggregates. They are also called associated colloids, e.g., soaps and detergents. They can form ions and may contain 100 or more molecules to form a micelle.
Question 26.
Explain the terms with suitable examples:
- Alcosol
- Aerosol
- Hydrosol.
Answer:
- Alcosol: It is a colloidal solution in which alcohol is the dispersion medium. For example, colloids which has cellulose nitrate as a dispersed phase and ethyl alcohol as the dispersion medium.
- Aerosol: It is a colloidal solution in which liquid is a dispersed phase and gas is a dispersion medium e.g., fog, mist, cloud, etc.
- Hydrosol: It is a colloidal solution in which solid is a dispersed phase and water is a dispersion, e.g., gold sol, arsenious sulphide sol, ferric oxide sol, etc.
Question 27.
Comment on the statement that colloid is not a substance but a state of substance’.
Answer:
Common salt (atypical crystalloid in an aqueous medium) behaves as a colloid in a benzene medium. Hence, we can say that a colloidal substance does not represent a separate class of substances. When the size of the solute particle lies between 1 nm and 1000 nm, it behaves as a colloid.
Hence, we can say that colloid is not a substance but a state of the substance which is dependent on the size of the particle.
A colloidal state is intermediate between a true solution and a suspension.
We hope the NCERT Solutions for Class 12 Chemistry Chapter 5 Surface Chemistry help you. If you have any query regarding NCERT Solutions for Class 12 Chemistry Chapter 5 Surface Chemistry, drop a comment below and we will get back to you at the earliest.