By going through these CBSE Class 12 Chemistry Notes Chapter 12 Aldehydes, Ketones and Carboxylic Acids, students can recall all the concepts quickly.
Aldehydes, Ketones and Carboxylic Acids Notes Class 12 Chemistry Chapter 12
→ Aldehydes and Ketones both contain carbonyl C = O group and hydrogen while in Ketones, it is bonded to two carbon atoms.
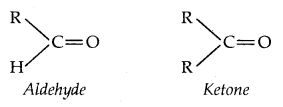
→ Carbon in both aldehydes and Ketones is sp2 hybridized:
If the Carbonyl group Carboxylic acid. is attached to -OH group, it is called Carboxylic Acid
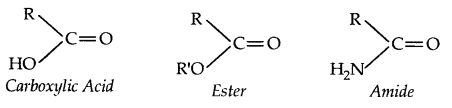
Common names:
IUPAC Names: The IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending – e with – al and – one respectively.
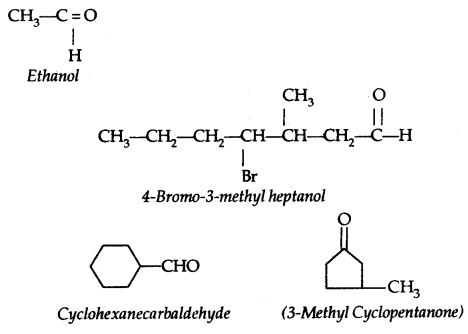
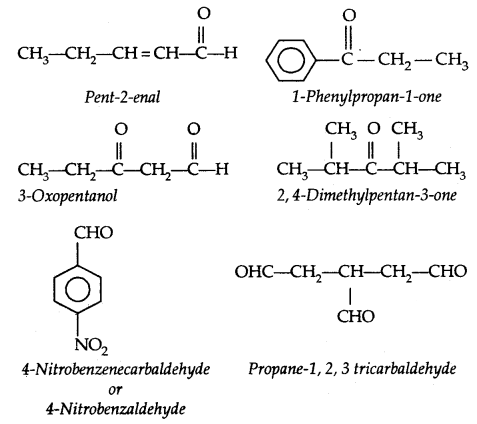
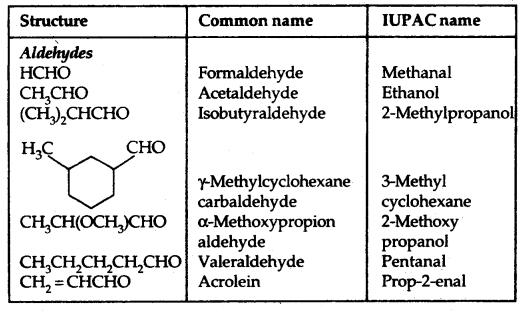
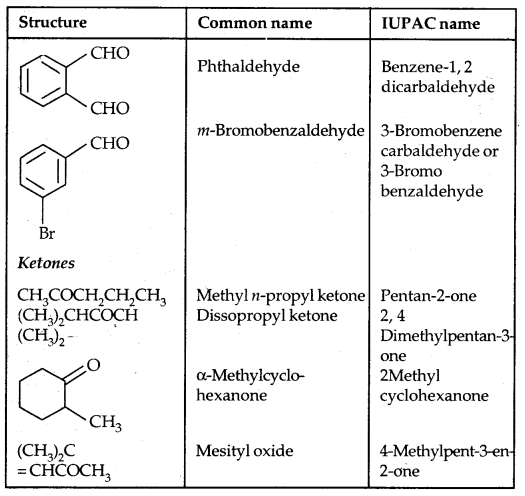
Table: Common and IUPAC Names of Some Aldehydes and Ketones:
→ Structures of the Carbonyl group): The carbonyl carbon atom is sp2 hybridized and forms three sigmas (σ) bonds.
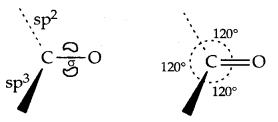
C = O is polarised due to the higher electronegativity of oxygen relative to carbon. Hence the carbonyl carbon is an electrophilic (Lewis acid) and carbonyl oxygen, a nucleophile (Lewis base) centre.
![]()
The carbonyl group is highly polar.
Preparation of Aldehydes and Ketones:
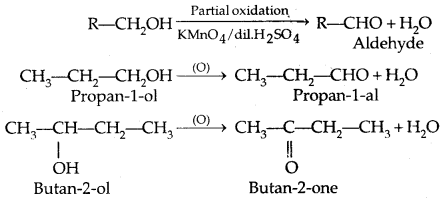
1. Aldehydes are prepared by the partial oxidation of primary alcohols while ketones are obtained from secondary alcoholic.
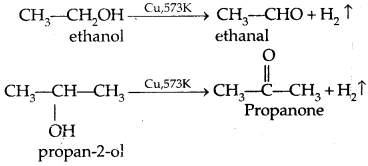
2. Catalytical dehydrogenation of primary alcohols with red hot Cu gauze at 573 K gives aldehydes and secondary alcohols give ketones.
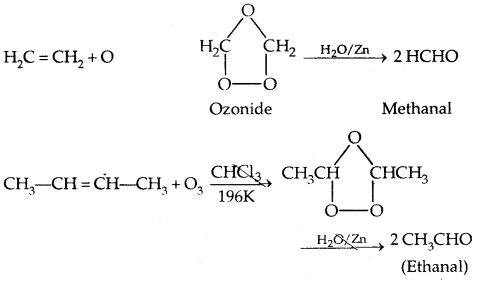
3. From ozonolysis of alkenes:
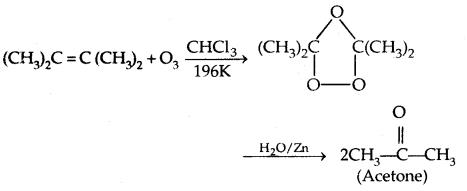
4. From Alkynes from hydration:
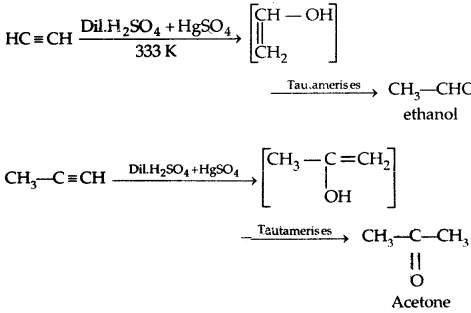
Preparation of Aldehydes only
1. Rosenmund’s Reaction: Reduction of acid Chlorides with H2 and Pd/BaS04 catalyst in boiling xylene solution.
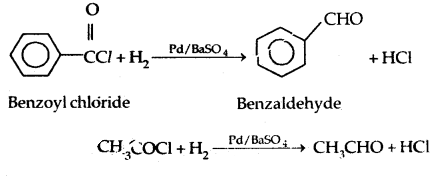
2. From Nitriles and esters: Stephen Reaction
![]()
Esters are reduced to aldehydes with DIBAL-H [diisobutyl- aluminium hydride]
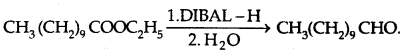
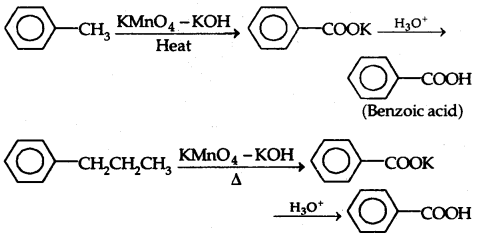
3. From Aromatic hydrocarbons:
(a) With chromyl chloride (Cr02Cl2): Etard’s Reaction
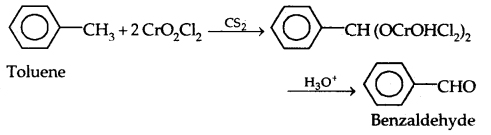
(b) With Chromic Oxide (Cr03)
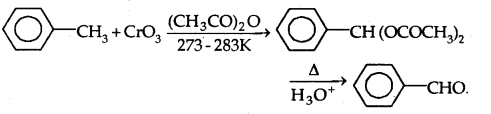
(c) By side-chain chlorination followed by hydrolysis
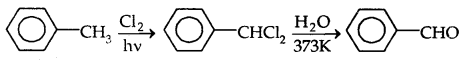
(d) By Gatterman-Koch Reaction
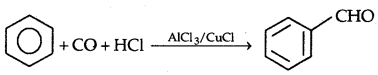
Methods of Preparation for Ketones:
1. From acid chlorides
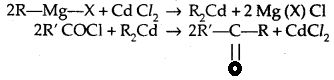
2. From nitriles
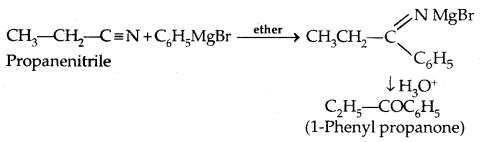
3. From benzene: By Friedel-Crafts acylation:
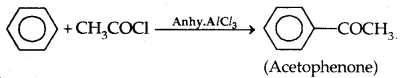
→ Physical Properties:
1. Methanal is a gas at room temperature. Ethanol is a volatile liquid. Other aldehydes and ketones are liquid or solid at room temperature.
2. B. Pts of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses due to weak molecular association in aldehydes and ketones due to dipole-dipole interactions. Their B.Pts. are lower than those of alcohols due to the absence of H-bonding.
The following compounds of molecular masses 58 and 60 are ranked in order of increasing boiling points.
| B.Pt (K) | M.Mass | |
| n-Butane | 273 | 58 |
| Methoxyethane | 281 | 60 |
| Propanal | 322 | 58 |
| Aoelone | 329 | 58 |
| Propan-1-ol | 370 | 60 |
3. The lower members of aldehydes and ketones such as methanal, ethanal and propanone are miscible with water in all proportions because they form H-bonds with water.
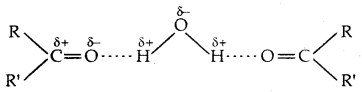
Solubility of aldehydes and ketones decreases rapidly on increasing the length of the alkyl group.
4. The lower aldehydes have a sharp pungent odour. As molecular mass increases, the odour becomes less pungent and more fragrant.
Chemical Reactions of Aldehydes and Ketones:
1. Nucleophilic addition Reactions: On the attack of a nucleophile on the carbonyl carbon, the hybridization of C changes from sp2 to sp3.
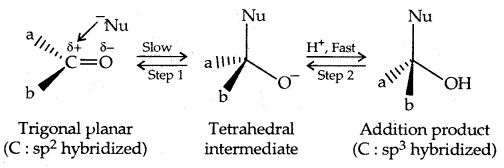
Aldehydes are more susceptible to nucleophilic addition reactions than ketones due to steric and electronic factors.
The reactivity of aldehydes and ketones towards nucleophilic addition reactions is
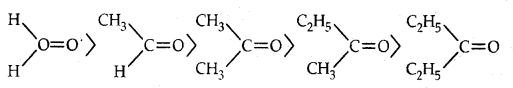
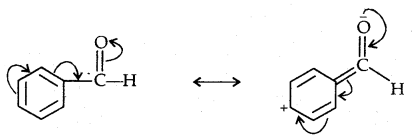
The polarity of the carbonyl group is reduced in benzaldehyde due to resonance and hence it is less reactive than propanal.
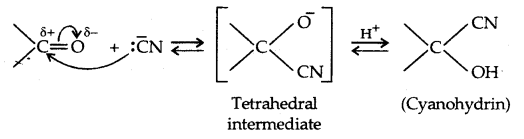
1. Addition of Hydrogen cyanide (HCN):
HCN + OH- ⇌ -CN + H2O
2. Addition of Sodium bisulphite (NaHSO3):
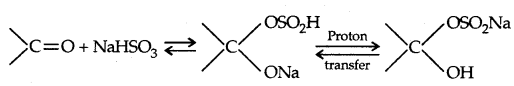
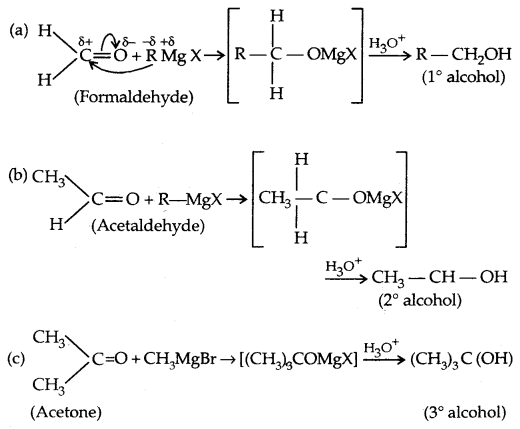
3. Addition of Grignard’s reagent: They give 1°, 2°, 3° alcohols.
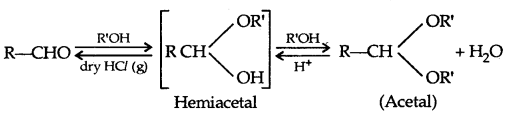
4. Addition of alcohols: Acetals/Ketals are formed
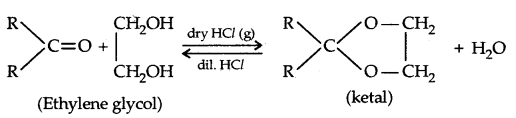
Ketones, under similar conditions, react with ethylene glycol to form cyclic products known as ethylene glycol ketals.
5. Addition of ammonia and its derivatives [Addition- Elimination].
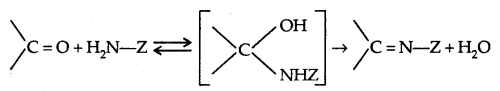
Z = alkyl, aryl, OH, NH2, C6H5NH, NHCONH2 etc.
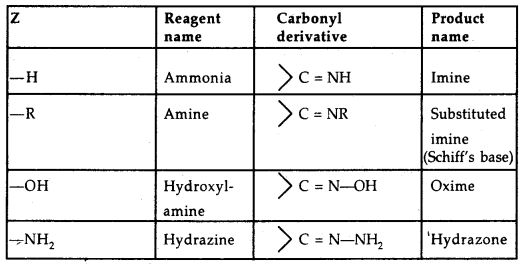
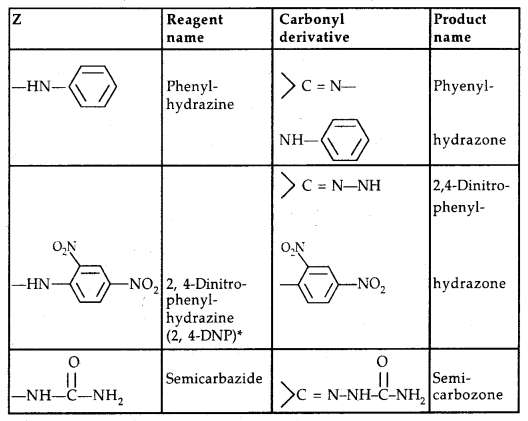
[Table: Some N-Substituted Derivatives of aldehydes and ketones
![]()
2, 4-DNP-derivatives are yellow, orange or red solids, useful for characterization of aldehydes and ketones.
2. Reduction:
1. Reduction to alcohols: Aldehydes and ketones are reduced to primary and secondary alcohols respectively by sodium borohydride (NaBH4) or lithium aluminium hydride (LiAlH4) as well as by catalytic hydrogenation.
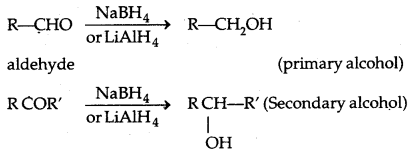
2. Reduction to hydrocarbons: The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with zinc-amalgam and concentrated hydrochloric acid [Clemmensen reduction) or with hydrazine followed by heating with sodium or potassium hydroxide in a high boiling solvent such as ethylene glycol (Wolff-Kishner reduction).
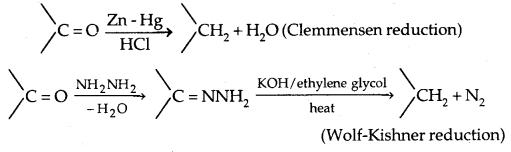
3. Oxidation: Aldehydes differ from ketones in their oxidation reactions,
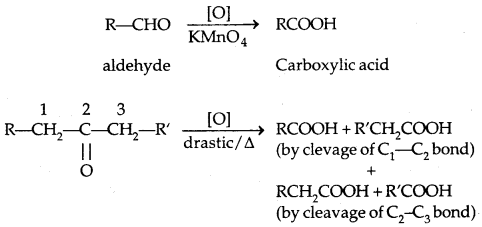
Due to the easy oxidation of aldehydes as compared to ketones, they can be distinguished from the following two steps:
1. Tollen’s test:
R—CHO + 2 [Ag(NH3)2]+ + 3 OH– → RCOO– + Ag (s) + 2 H2O + 4 NH3
On warming an aldehyde with freshly prepared ammonical AgNO3 solution (Tollen’s reagent), a bright silver mirror is produced.
2. Fehling’s Test: On heating an aldehyde with Fehling reagent, a reddish-brown precipitate is obtained.
RCHO + 2 Cu2+ + 5 OH– → RCOO– + Cu2O + 3 H2O. (red-brown ppt.)
→ Oxidation of methyl ketones by haloform reaction: All those carboxyl compounds containing the – COCH3 group respond to this test.
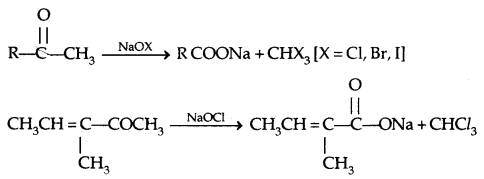
If NaOI is used, yellow ppt. of CHI3 is formed which is used to detect the CH3C = O group.
4. Reactions due to α-hydrogen atom: α-hydrogens in aldehydes and ketones are acidic in nature due to the strong electron-withdrawing effect of the carbonyl group and resonance stabilisation of the conjugate base.
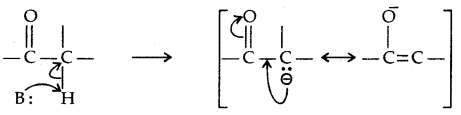
1. Aldol Condensation: Aldehydes and ketones having at least one α-hydrogen undergo Aldol Condensation in the presence of dil. alkali to form β-hydroxy aldehydes (aldol) or β-hydroxy ketones [ketol] respectively.
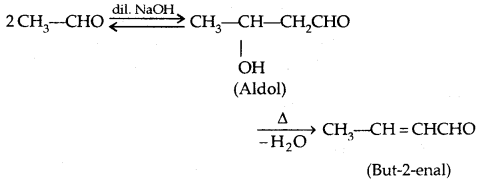
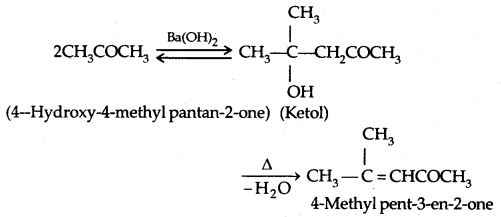
2. Cross Aldol Condensation: When aldol condensation is carried out between two different aldehydes and/or ketones, it is called Cross aldol condensation.
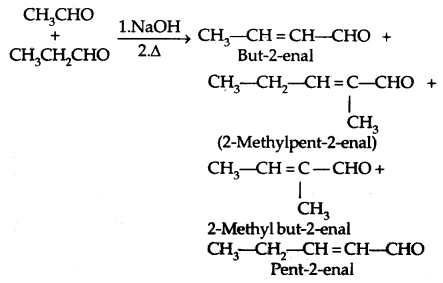
Ketones can also be used as one component in the cross aldol condensation.
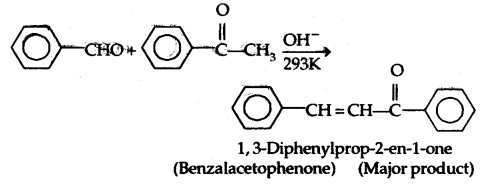
5. Other Reactions:
1. Cannizzaro Reactions: Aldehydes that do not have an a- hydrogen atom, undergo self oxidation-reduction called Disproportionation reactions on treatment with concentrated alkali.
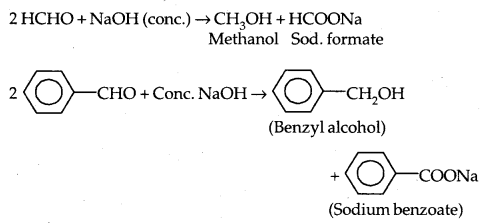
2. Electrophilic substitution reactions: Aromatic aldehydes and ketones undergo electrophilic substitution reactions at the ring and the carbonyl group acts as a deactivating and meta-directing group.
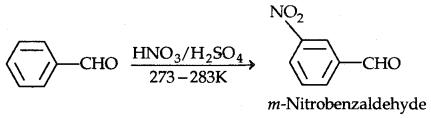
Uses of aldehydes and ketones
- Formalin (aqueous solution of formaldehyde) is used to preserve biological specimen and to prepare bakelite.
- Acetaldehyde is used to prepare acetic acid, ethyl acetate, vinyl acetate, polymers and drugs.
- Benzaldehyde is used in perfumery and in dye industries.
- Acetone and ethyl methyl ketone are common industrial solvents.
II. Carboxylic Acids: Carbon compounds containing a carboxylic -COOH group are called Carboxylic acids.
→ Nomenclature and Structure of Carboxylic Group: In the IUPAC system last – e of alkanes is replaced with – oic acid. For naming compounds containing more than one carboxylic group, the ending – C of the alkane is retained.
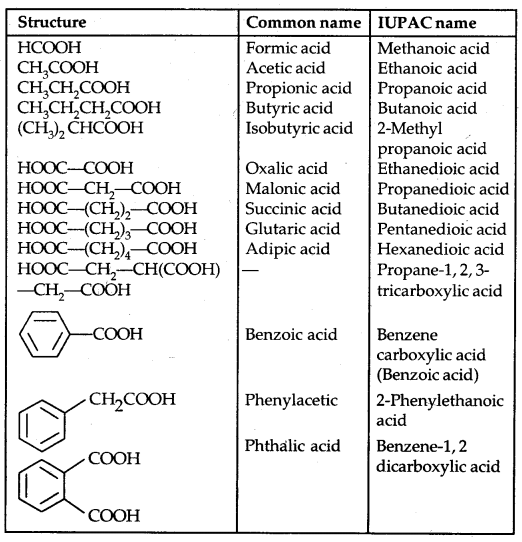
Table: Names and Structures of Some Carboxylic Acids:
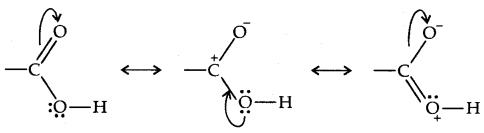
→ Structure of Carboxylic Group: Because of the possible resonance structure shown below, the carboxylic carbon is less electrophilic. Then carbonyl carbon and the bonds of the carboxylic carbon lie in the plane and are separated by 120°.
Methods of Preparation of Carboxylic Acids:
1. From Primary Alcohols and Aldehydes: Primary
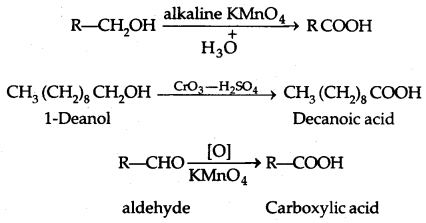
2. From Alkyl benzenes:
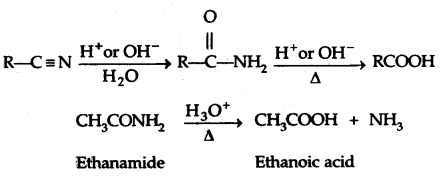
3. From Nitriles and amides: By Hydrolysis
2. Due to extensive hydrogen bonding, carboxylic acid molecules get associated and thus have higher boiling points as compared to aldehydes, ketones and even alcohols of comparable molecular masses.
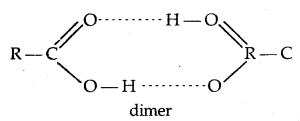
(In vapour state are in an aprotic solvent.)
3. Simple aliphatic carboxylic acids having up to four C atoms are miscible in water the formation of hydrogen bonds with water.
The solubility decrease with an increasing number of carbon atoms.
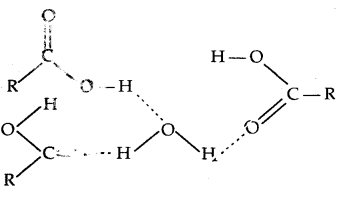
(Hydrogen bonding of RCOOH with H2O)
Chemical Reaction:
Reactions involving cleavage of O-HBond
Acidic character Reactions with metals and alkalies:
2RCOOH + 2 Na → 2 RCOO– Na+ + H2 Sodium carboxylate
RCOOH + NaOH → RCOO– Na+ + H2O
RCOOH + NaHCO3 → RCOO– Na+ + H2O + CO2
Carboxylic acid dissociates in water to give resonance-stabilized carboxylate anion and the hydronium ion.
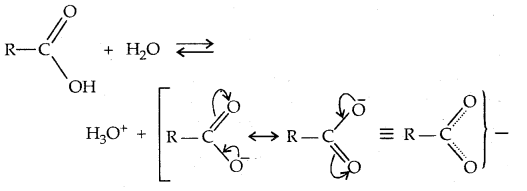
For the above reaction:
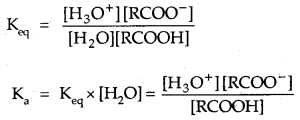
Where Keq, is the equilibrium constant and Ka is the acid dissociation constant.
pka = – log ka
The pka of hydrochloric acid is – 7.0, whereas pka of trifluoracetic acid (tine strongest organic acid), benzoic acid, and acetic acid are 0.23, 4.19 and 4.76 respectively.
Smaller the pka, the stronger the acid.
Carboxylic acids are weaker than mineral acids, but they are stronger acids than alcohols and many simple phenols [pka for phenols is ~ 10 and for ethanol ~ 16). Carboxylate anion is more resonance stabilized than phenoxide ion as the – ve charge is spread on two oxygen atoms rather than one in phenoxide ion.
Effect of Substituents on the acidity of carboxylic acids: Electron withdrawing groups increase the acidity of carboxylic acids by stabilizing the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects. Conversely, electron-donating groups decrease the acidity by destabilizing the conjugate base.
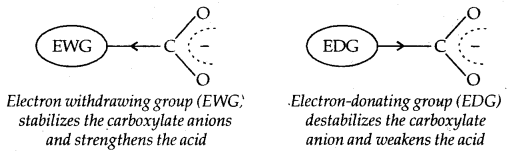
The effect of the following groups in increasing acidity order is Ph < I < Br < Cl < F < CN < NO2 < CF3.
Thus the following acids are arranged in order of increasing acidity/based on pka values.
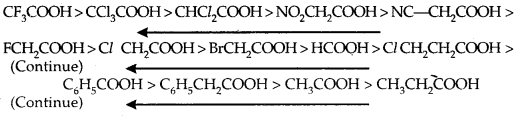
Direct attachment of groups such as phenyl or vinyl to the carboxylic acid, increases the acidity of corresponding carboxylic acid, contrary to the decrease expected due to the resonance effect shown below:
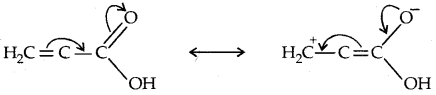
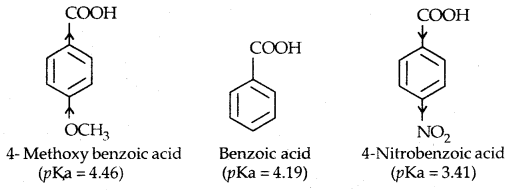
This is because of the greater electronegativity of sp2 hybridised carbon to which carboxyl carbon is attached. The presence of an electron-withdrawing group on the phenyl of the aromatic carboxylic acid increases their acidity while electron-donating groups decrease their acidity.
Reactions Involving Cleavage of C—OH Bond:
1. Formation of Anhydride
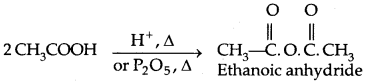
2. Esterification:
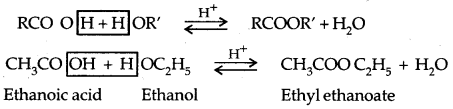
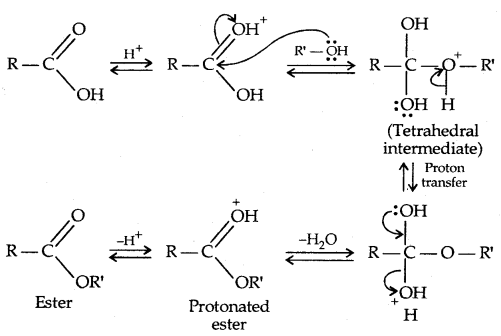
Mechanism of Esterification:
3. Reaction involving PCl5 PCl3 SOCl2: Thionyl chloride (SOCl2) is preferred as the other two products are gases.
RCOOH + PCl5 → R COCl + POCl3 + HCl
3RCOOH + PCl3 → 3 ROCl + H3PO3
RCOOH + SOCl2 → RCOCl + SO2 ↑ + HCl ↑.
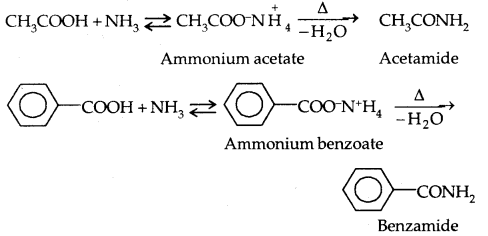
4. Reactions with ammonia:
Reactions Involving: COOH Group:
1. Reduction:
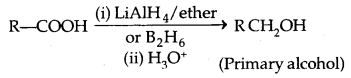
2. Decarboxylation:
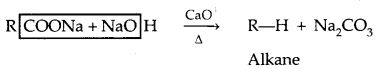
Kolbe’s Electrolysis
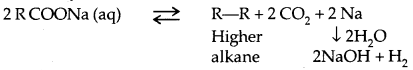
Substitution reactions in the hydrocarbon part:
1. Halogenation: Hell-Volhard-Zelinsky Reaction
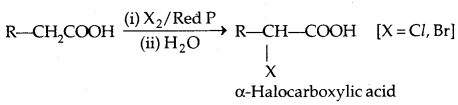
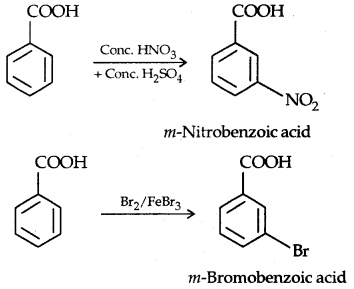
2. Ring Substitution: —COOH group present in the ring is a deactivating group and meta-directing group. They do not, however, undergo Friedel-Craft reaction.
It is because the carboxylic group (— COOH) is a deactivating group and the catalyst aluminium chloride (Lewis acid) gets bonded to the carboxylic group.
Uses of Carboxylic acids: Methanoic acid is used in rubber, textile, dyeing, leather and electroplating industries. Ethanoic acid is used as a solvent and as vinegar in the food industry. Hexanedioic acid is used in the manufacture of nylon-66. Esters of benzoic acid are used in perfumery. Sodium benzoate is used as a food preservative. Higher fatty acids are used for the manufacture of soaps and detergents.