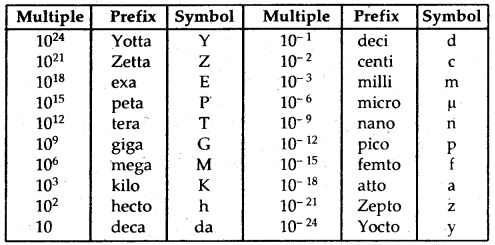
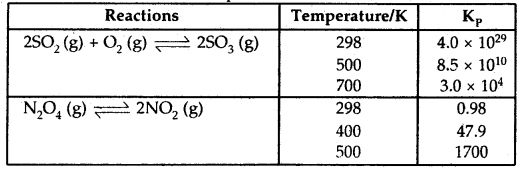
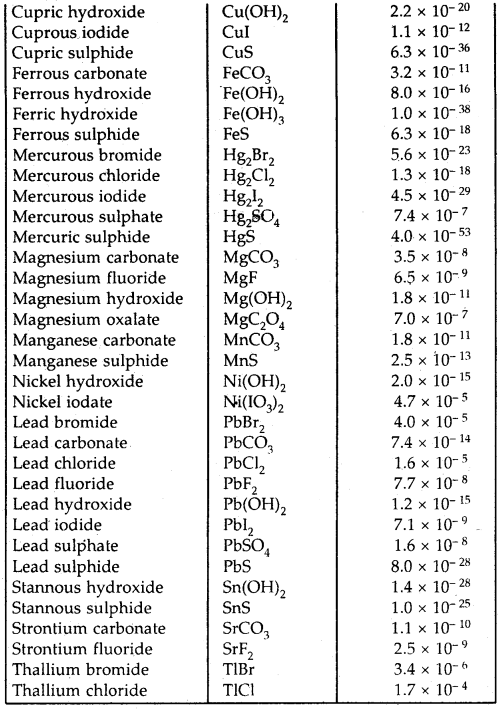
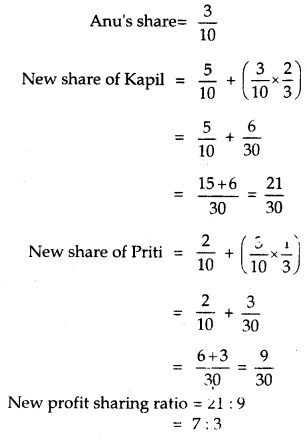
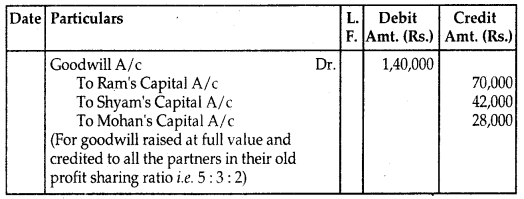
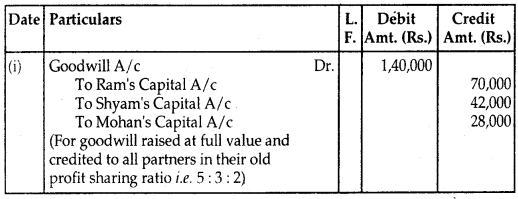
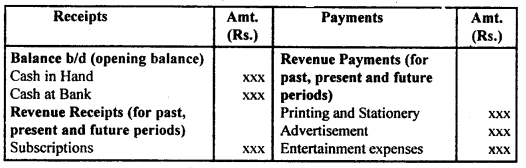
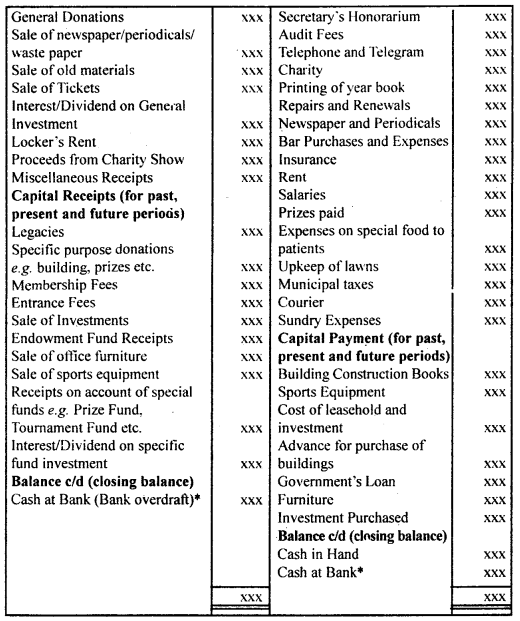
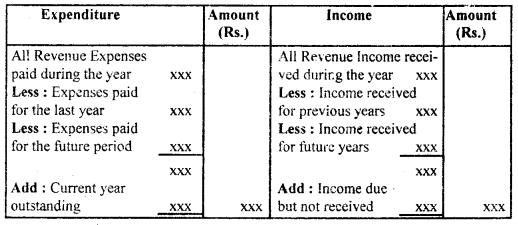
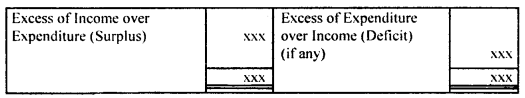
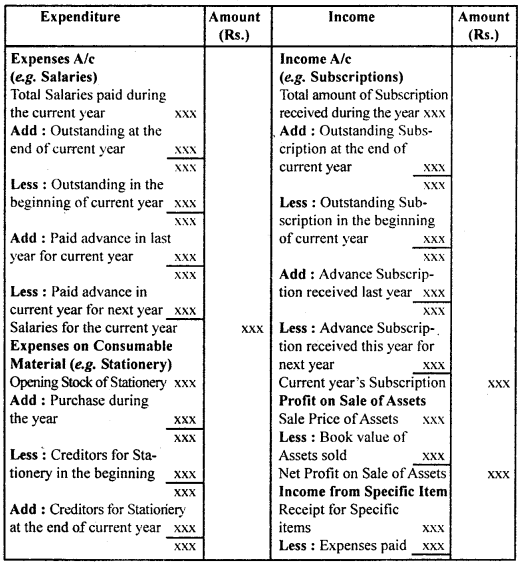
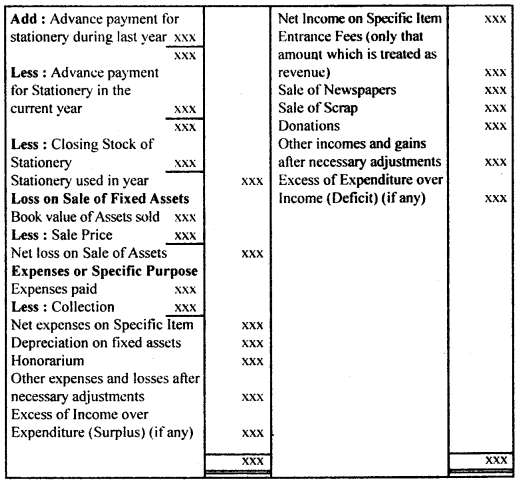
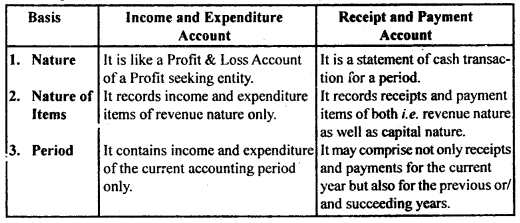
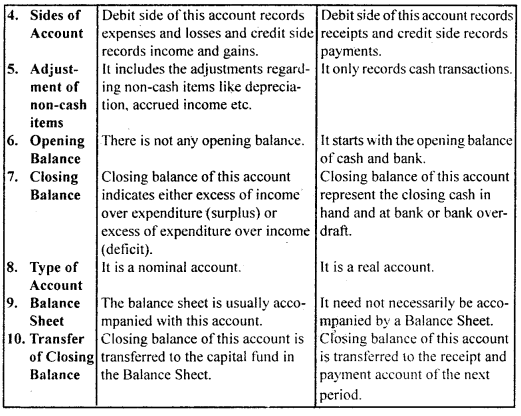
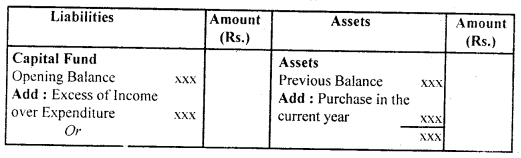
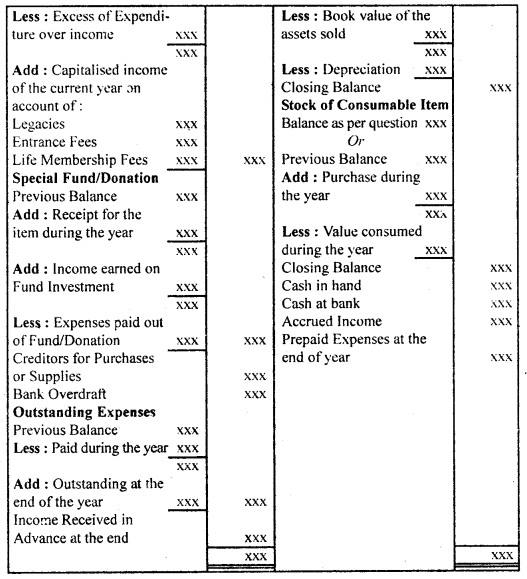
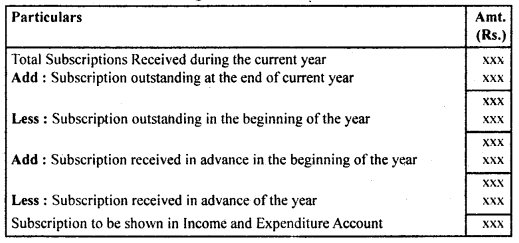
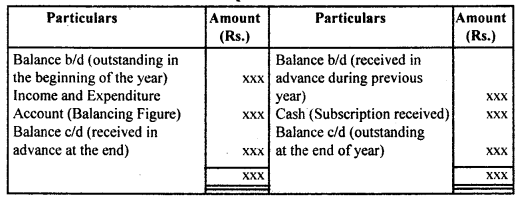
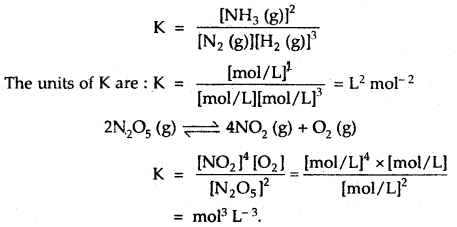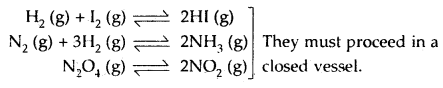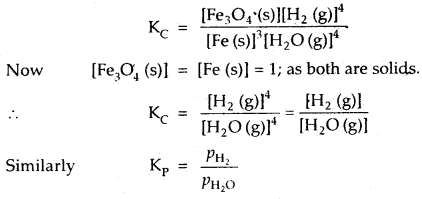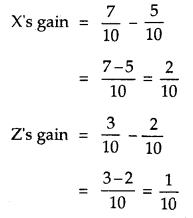By going through these CBSE Class 12 Hindi Notes Chapter 9 रुबाइयाँ, गज़ल Summary, Notes, students can recall all the concepts quickly.
रुबाइयाँ, गज़ल Summary Notes Class 12 Hindi Aroh Chapter 9
रुबाइयाँ, गज़ल कविता का सारांश
‘रुबाइयाँ’ फ़िराक गोरखपुरी द्वारा रचित काव्य है जो उनके काव्य-संग्रह ‘गुले नगमा’ से संग्रहित है। इस कविता में कवि ने वात्सल्य रस का अनूठा चित्रण प्रस्तुत किया है। यह वात्सल्य भावना से ओत-प्रोत कविता है। माँ अपने चाँद के टुकड़े को अपने आँगन में खड़ी । होकर अपने हाथों में झुला रही है। माँ अपने नन्हें बच्चे को अपने आँचल में भरकर बार-बार हवा में उछाल देती है जिससे नन्हें बच्ची । की हँसी सारे वातावरण में गूंज उठती है। माँ अपने बच्चे को निर्मल जल से नहलाती है।
उसके उलझे बालों को कंघी से संवारती है। बच्चा भी माँ को बड़े प्यार से देखता है जब माँ अपनी गोदी में लेकर उसे कपड़े पहनाती है। दीवाली के अवसर पर संध्या होते ही घर पुते और सजे हुए दिखते हैं। घरों में चीनी मिट्टी के चमकते खिलौने सुंदर मुख पर नई चमक ला देते हैं। माँ प्रसन्न होकर अपने नन्हें बच्चे द्वारा बनाए मिट्टी के घर में दीपक जलाती है। बच्चा अपने आँगन में ठिनक रहा है।
वह ठिनकता हुआ चाँद को देखकर उस पर : मोहित हो जाता है। बच्चा चंद्रमा को माँगने की हठ करता है तो माँ दर्पण में उसे चाँद उतारकर दिखाना चाहती है। रक्षा-बंधन एक रस का बंधन है। सावन मास में आकाश में हल्के-हल्के बादल छाए हुए हैं। राखी के कच्चे धागों पर लगे लच्छे बिजली के समान चमकते हैं। कवि कहता है कि सावन का जो संबंध घटा से है, घटा का जो संबंध बिजली से है, वही संबंध भाई का बहन से है। इसी बिजली के समान चमकते लच्छेदार कच्चे धागे को बहन अपने भाई की कलाई में बाँधती है।
रुबाइयाँ, गज़ल कवि परिचय
कवि-परिचय जीवन-परिचय-फ़िराक गोरखपुरी उर्दू-फ़ारसी के महान शायर थे। उनका जन्म 28 अगस्त, 1896 ई० को उत्तर प्रदेश के गोरखपुर में हुआ था। उनका मूल नाम रघुपति सहाय फ़िराक था। उन्होंने रामकृष्ण की कहानियों से अपनी शिक्षा की शुरुआत की। बाद में अरबी, फ़ारसी और अंग्रेजी में शिक्षा ग्रहण की। 1917 ई० में डिप्टी कलक्टर के पद पर नियुक्त गए लेकिन स्वराज आंदोलन से प्रेरित होकर 1918 ई० में पद त्याग दिया। 1920 ई० में इन्होंने स्वाधीनता आंदोलन में बढ़-चढ़कर भाग लिया जिसके कारण इन्हें डेढ़ वर्ष की जेल भी हुई। उन्होंने इलाहाबाद विश्वविद्यालय के अंग्रेजी विभाग में अध्यापक के पद पर कार्य किया।

उन्हें ‘गुले नगमा’ के लिए साहित्य अकादमी, ज्ञानपीठ पुरस्कार तथा सोवियत लैंड नेहरू अवार्ड से सम्मानित किया गया। अंततः सन् 1983 में ये अपनी महान शायरी संसार को सौंप कर स्वर्ग सिधार गए। रचनाएँ-गोरखपुरी जी ने शायरी के क्षेत्र में नए विषयों का पदार्पण कर प्राचीन समय से चली आ रही परंपरा को तोड़ा। अनेक नए विषयों की खोज कर उन्होंने अनेक रुबाइयाँ, गज़ले आदि लिखीं। इनकी महत्त्वपूर्ण कृतियाँ निम्नलिखित हैंगुले नगमा, बज्मे जिंदगी, रंग-ए-शायरी, उर्दू गजल, गोई आदि। साहित्यिक विशेषताएँ-फ़िराक गोरखपुरी उर्दू साहित्य के महान शायर माने जाते हैं। इनकी शायरी की प्रमुख विशेषताएँ निम्नलिखित हैं
(i) नवीन विषयों का चित्रण-उर्दू शायरी का साहित्य रूमानियत, रहस्य और शास्त्रीयता से बँधा रहा है। फ़िराक गोरखपुरी और नजीर आदि । साहित्यकारों ने इस परंपरा को तोड़ा। फिराक ने परंपरागत भाव-बोध और शब्द-भंडार का उपयोग करते हुए उसे नए विषयों से जोड़ा। उन्होंने उर्दू शायरी को रहस्य, रूमानियत और शास्त्रीयता से बाहर निकालकर सामाजिक दुख-दर्द के साथ जोड़कर प्रस्तुत किया।
(ii) वैयक्तिकता का चित्रण-फ़िराक गोरखपुरी ने उर्दू शायरी में व्यक्तिगत अनुभूति की ओर मोड़ा और उन्होंने अपनी शायरी के । माध्यम से अपने सुख-दुखों का चित्रण किया, जैसे
तेरे गप का पासे अदब है कुछ दुनिया का ख्याल भी है।
सबसे छिपा के दर्द के मारे चुपके-चुपके रोते हैं।
(iii) शृंगार वर्णन-गोरखपुरी की शायरी में श्रृंगार रस का भी चित्रण हुआ है। इनकी शायरी में श्रृंगार के दोनों पक्षों अर्थात संयोग और । वियोग का वर्णन मिलता है लेकिन संयोग की अपेक्षा विरहावस्था का अधिक चित्रण हुआ है। इनके साहित्य में प्रेमी-प्रेमिका के। वियोग पक्ष की सजीव एवं मार्मिक अभिव्यक्ति हुई है।
ऐसे में त याद आए है अंजुमने भय में रिन्दों को।
रात गये गर्दू पै फरिश्ते बाबे गुनह जग खोले हैं।
(iv) वात्सल्य रस का चित्रण-गोरखपुरी की शायरी में वात्सल्य रस की अनुपम अभिव्यक्ति हुई है। वात्सल्य रस का चित्रण करते हुए ऐसा लगता है मानो कवि एक माँ का हृदय पा गया हो। माँ का बच्चे के प्रति प्रेम तथा अपने नन्हें बच्चे के प्रति माँ की वात्सल्य अनुभूतियों का सजीव अंकन किया है। कवि वात्सल्य रस की छोटी-से-छोटी अनुभूति का भी सजीव वर्णन किया है। नहला के छलके-छलके निर्मल जल से उलझे हुए गेसुओं में कंघी करके किस प्यार से देखता है बच्चा मुँह को जन घटनियों में ले के है पिन्हाती कपड़े।
(v) भारतीय संस्कृति का अनूठा चित्रण-फ़िराक गोरखपुरी एक शायर होने से पहले एक सच्चे भारतीय थे। वे हिंदू-मुस्लिम आदि | संकीर्णताओं से दूर एक मानव थे, इसीलिए उन्होंने धार्मिक और सामाजिक सहिष्णुता पर बल दिया। उन्होंने भारतीय संस्कृति, तीज-त्योहार आदि का अनूठा चित्रण किया है। गोरखपुरी ने अपनी रुबाइयों में दीवाली, रक्षा-बंधन आदि त्योहारों की सजीव अभिव्यक्ति की है। जैसे
रक्षा-बंधन की सुबह रस की पुतीली
छायी है घटा गगन की हलकी-हलकी
बिजली की तरह चमक रहे हैं लच्छे
भाई के है बाँधती चमकती गखी॥
(vi) देशभक्ति की भावना-फ़िराक गोरखपुरी एक सच्चे देशभक्त थे। सन् 1920 ई० में भारतीय स्वाधीनता आंदोलन में भाग लेने के कारण वे जेल भी गए थे। यही देशभक्ति की भावना उनके साहित्य में भी दृष्टिगोचर होती है। उन्होंने अपनी शायरी के माध्यम से भारतीय समाज को जागृत करने का प्रयास किया है।
(vi) प्रकृति चित्रण-गोरखपुरी जी प्रकृति से भी प्रेम करते थे जो उनके अनूठे प्रकृति सौंदर्य में देखने को मिलता है। उन्होंने अपने | साहित्य में वसंत ऋतु, बाग, उद्यान, टिमटिमाते तारे, फूलों की महक आदि का सजीव और मनोहारी चित्रण किया है। नौरस गुंचे पंखड़ियों की नाजुक गिरहें खोले हैं या उड़ जाने को रंगों बू गुलशन में पर तोले हैं। तारे आँखें झपकावे हैं ज़र्रा-जर्रा सोये हैं तुम भी सुना हो यारो ! शब में सन्नाटे कुछ बोले हैं।
(vi) भाषा-शैली-गोरखपुरी जी उर्दू के श्रेष्ठ शायर हैं। उन्होंने अपनी शायरी को अभिव्यंजना प्रदान करने के लिए नई भाषा और नए शब्द-भंडार का प्रयोग किया है। इन्होंने अपनी भाषा में उर्दू, फारसी, साधारण बोलचाल, खड़ी हिंदी बोली आदि का प्रयोग किया है। ये लाक्षणिक प्रयोगों और चुस्त मुहावरों के लिए प्रसिद्ध हैं। इन्होंने भी ‘मौर और गालिब’ की तरह कहने की शैली को साधकर। साधारण मनुष्य के रूप में अपनी बात कही है।
(ix) अलंकार-छंद का चित्रण-फ़िराक ने अपनी शायरी में शब्दालंकार तथा अर्थालंकार दोनों प्रकार के अलंकारों का प्रयोग किया है। इनकी शायरी में अनुप्रास, पदमैत्री, स्वरमैत्री, उपमा, उत्प्रेक्षा, मानवीकरण, संदेह आदि अलंकारों का प्रयोग हुआ है। वस्तुतः फ़िराक गोरखपुरी शायरी-साहित्य के संसार में चमकता हुआ सितारा थे। उनका उर्दू शायरी में महान योगदान है।