The reaction of Acids With Bases:
Acids react with bases to form salt and water. This is known as neutralization reaction as the effect of a base is nullified by acid and vice-versa.
Example: NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Neutralization Reaction:
The reaction between an add and a base to give salt and water is known as a neutralization reaction. In general, a neutralization reaction can be written as
Acid + Base → Salt + Water
Example 1.
10 mL of a solution of NaOH is found to be completely neutralized by 8 mL of a given solution of HCl. If we take 20 ml of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralize it will be
(a) 4 mL
(b) 8 mL
(c) 12 mL
(d) 16 mL
Answer:
(d) 16 mL
Explanation: As 10 mL of NaOH solution is completely neutralized by 8 mL of HCl solution, 16 mL of HCl solution will be required to neutralize 20 mL of NaOH solution.
![]()
Similarities among Acids and Bases:
All acids have similar chemical properties. All acids generate hydrogen gas on reacting with metals, so hydrogen is common to all acids.
Acids produce hydrogen ions, H+(aq), in solution, which are responsible for their acidic properties.
Solutions of acids and bases conduct electricity as both acids and bases dissociate into hydrogen ions (H+) and hydroxide ions (OH–) respectively, which are responsible for the conduction of electric current through the solution.
Example 2.
Case-Based:
Take solutions of glucose, alcohol, hydrochloric acid, sulphuric acid, etc. Fix two nails on a cork, and place the cork in a 100 mL beaker. Connect the nails to the two terminals of a 6-volt battery through a bulb and a switch, as shown in Fig. below.
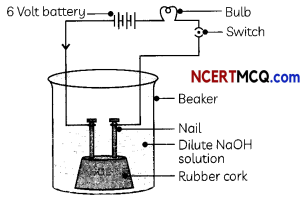
Now pour some dilute HCl into the beaker and switch on the current. Repeat with other solutions one by one.
(A) Four students recorded the following observations. Identify the correct observation:
| Electrolyte | Observation |
| (a) Dilute HCl | The bulb does not glow |
| (b) Glucose | Bulb glows |
| (c) Dilute NaOH | Bulb glows |
Answer:
(c) Electrolyte: Dilute HCl; Observation: Bulb glows
Explanation: The bulb glows when the electrolyte is an acid or a base as in both cases ions are produced in an aqueous solution due to which current flows through the solution.
(B) The activity is repeated with alcohol as the electrolyte. Which of the following statement(s) is (are) correct?
(I) Bulb will not glow because the electrolyte is not acidic.
(II) Bulb will glow because alcohol contains Hydrogen and provides ions for the conduction of electricity.
(III) Bulb will not glow because alcohol does not provide ions for the conduction of electricity.
(IV) Bulb will glow because it depends upon the type of electrolytic solution.
(a) (I) and (III)
(b) (II) and (IV)
(c) (III) only
(d) (IV) only
Answer:
(c) (III) only
(C) Why do HCl, HNO3, etc., show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer:
Solutions like HCl, HNO3, etc. show acidic characters in aqueous solutions as they contain the cation H+ and produce hydrogen ions, H+(aq) in solution. Whereas solutions of compounds such as alcohol and glucose do not form any such ions and hence they do not show acidic character.
(D) Why does an aqueous solution of acid conduct electricity?
Answer:
An aqueous solution of an acid conducts electricity as acids dissociate into hydrogen ions (H+) in the solution, which is responsible for the conduction of electricity through the solution.
(E) Assertion: An aqueous solution of glucose conducts electricity.
Reason: Electric current is carried through the solution by ions.
(a) Both (A)and R are true, and (R) is the correct explanation of the assertion (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Answer:
(d) (A) is false, but (R) is true.
Explanation: An aqueous solution of glucose does not conduct electricity as glucose does not dissociate into ions even in solution. Electric current will flow in a solution only by ions, as happens in the case of an aqueous solution of acids and bases.
![]()
Example 3.
Why does distilled water not conduct electricity, whereas rainwater does?
Answer:
Electric current is conducted in solutions by ions. As distilled water does not contain any ions, it does not conduct electricity. However, rainwater conducts electricity due to the presence of small amounts of acidic oxides in rainwater such as SO2, NO2 which make It a better conductor of electricity.
Acid and Bases in Water Solution:
Hydrogen or Hydronium Ions
All acids produce hydrogen ions (H+) only in presence of water. The separation of H+ ions from HCl molecules cannot occur in the absence of water.
HCl + H2O → H3O+ + Cl–
Hydrogen ions cannot exist alone, but they exist after combining with water molecules. Thus hydrogen ions must always be shown as H+(aq) or hydronium ion (H3O+).
H+ + H2O → H3O+
Hydroxide Ions:
Bases produce hydroxide ions (OH–) in water. Example: When sodium hydroxide is dissolved in water, it dissociates to form Na+ ions and OH– ions. NaOH(aq) → Na (aq) + OH
Alkalies:
All bases are not soluble in water. The bases which are soluble in water are called alkalis.
![]()
Example 4.
How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved in a solution of sodium hydroxide?
Answer:
A solution of sodium hydroxide is strongly basic due to the formation of hydroxide ions (OH-).
NaOH(s) → Na (aq) + OH (aq)
When the excess base is dissolved in such a solution, the concentration of hydroxide ions will increase further.
Explanation of Neutralization Reactions:
Neutralization reaction between acids and bases can be explained in terms of hydrogen ions produced by acids and hydroxide ions produced by bases as follows:
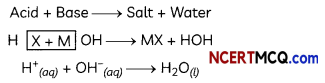
Dilution of Acids and Bases:
When an acid or a base is added to water, lot of heat is produced. The process of dissolving an acid or a base in water is a highly exothermic reaction.
The process of mixing an acid or base with water results in a decrease in the concentration of ions (H3O+/OH–) per unit volume and this process is called dilution. The acid or the base is said to be diluted.
The acid must always be added slowly to water with constant stirring. If water is added to a concentrated
acid, the heat generated may cause the mixture to splash out and cause burns.
Example 5.
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Answer:
Dilution of concentrated acid is a highly exothermic process. If we add water to the concentrated acid, the mixture may splash and cause severe burn injuries due to a large amount of heat produced. It is therefore recommended that acid should be added to the water drop by drop with constant stirring so that the heat evolved may be absorbed by the water.
![]()
Example 6.
How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
Answer:
When a solution of an acid is diluted by adding acid to the water, the concentration of hydronium ions decreases due to the increase in the volume of the solution. As the number of hydronium ions remains the same on diluting the acid, their concentration decreases.