Learninsta presents the core concepts of Biology with high-quality research papers and topical review articles.
Absorption Spectrum and Action Spectrum
The term absorption refers to complete retention of light, without reflection or transmission. Pigments absorb different wavelengths of light. A curve obtained by plotting the amount of absorption of different wavelengths of light by a pigment is called its absorption spectrum.
- Chlorophyll ‘a’ and chlorophyll ‘b’ absorb quanta from blue and red region.
- Maximum absorption peak for different forms of chlorophyll ‘a’ is 670 to 673, 680 to 683 and 695 to 705nm.
- Chlorophyll ‘a’ 680 (P680) and Chlorophyll ‘a’ 700 (P700) function as trap centre for PS II and PS I respectively.
Action Spectrum
The effectiveness of different wavelength of light on photosynthesis is measured by plotting against quantum yield. The curve showing the rate of photosynthesis at different wavelengths of light is called action spectrum.
From the graph showing action spectrum, it can be concluded that maximum photosynthesis takes place in blue and red region of the spectrum. This wavelength of the spectrum is the absorption maxima for Chlorophyll (a) and Chlorophyll (b). The Action Spectrum is instrumental in the discovery of the existence of two photosystems in O2 evolving photosynthesis (Figure 13.7).
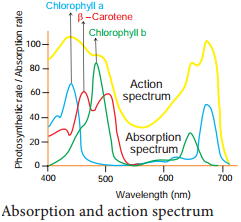
Absorption spectrum deals with wavelengths of light absorbed by each pigment whereas the action spectrum indicates the overall rate of photosynthesis at each wavelength of light. It shows the overall rate of photosynthesis at each wavelength of light.
An absorption spectrum defines the spectrum of electromagnetic radiation, or light, plants absorb. An action spectrum defines the spectrum of electromagnetic radiation most effective for photosynthesis. In other words, it is the part of the light spectrum that does the work.
The action spectrum for photosynthesis shows which wavelengths are used by plants to create energy, while the absorption spectrum shows which wavelengths are most absorbed by a specific molecule. But other molecules play a part as well, which is why there is some difference in the absorption and action spectra.
An action spectrum is a graph of the rate of biological effectiveness plotted against wavelength of light. It shows which wavelength of light is most effectively used in a specific chemical reaction. Some reactants are able to use specific wavelengths of light more effectively to complete their reactions.
Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet-visible spectroscopy are particularly common in analytical applications.
The similarity of the action spectrum of photosynthesis and the absorption spectrum of chlorophyll tells us that chlorophylls are the most important pigments in the process. The spectra are not identical, though, because carotenoids, which absorb strongly in the blue, play a role as well.
Absorption Spectrum. Definition. The range of a pigment’s ability to absorb various wavelengths of light. A graph plotting light absorption of a pigment versus wavelength.
An action spectrum is measured by plotting a response to light such as oxygen evolution, as a function of wavelength. If the pigments used to obtain the absorption spectrum are the same as those that cause the response, the absorption and action spectra will match.
Because more absorption leads to more action, peaks in an absorption spectrum will have corresponding peaks in an action spectrum. Thus, if a pigment can be found that has an absorption spectrum that matches a process’s action spectrum, it is likely that pigment is the photoreceptor for that process.
The pattern of absorption lines in a spectrum is diagnostic of the types of atoms and molecules present, for example, in the surface layers of a star or the atmosphere of a planet. Absorption lines are seen in the spectra of the Sun and other stars.
To get an absorption spectrum, just shine white light on a sample of the material that you are interested in white light is made up of all the different wavelengths of visible light put together. In the absorption spectrum there will be gaps.
An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies; since the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) in the spectrum.
The action spectrum for photosynthesis is much broader than the absorption spectrum of chlorophyll a. This is because accessory pigments with different absorption spectra also present in chloroplasts broaden the spectrum of colors that can be used for photosynthesis.