 On this page, you will find Acids Bases and Salts Class 10 Notes Science Chapter 2 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 2 Acids Bases and Salts will seemingly help them to revise the important concepts in less time.
On this page, you will find Acids Bases and Salts Class 10 Notes Science Chapter 2 Pdf free download. CBSE NCERT Class 10 Science Notes Chapter 2 Acids Bases and Salts will seemingly help them to revise the important concepts in less time.
CBSE Class 10 Science Chapter 2 Notes Acids Bases and Salts
Acids Bases and Salts Class 10 Notes Understanding the Lesson
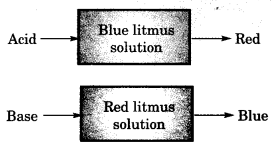
1. Acids and Bases: Acids are sour in taste and change the colour of blue litmus to red. The term has been derived from the Latin word ‘acidus’ which means sour taste. Generally acids have atleast one or more hydrogen atoms in their formulae.
An acid may be defined as a chemical substance which releases one or more H+ or HsO+ ions in aqueous solution.
For example, HCl, HNO3, H2SO4, etc.
Bases are bitter and change the colour of the red litmus to blue. Generally bases have one or more hydroxyl (OH–) groups. They produce hydroxyl ions (OH–) when dissolved in water.
A base may be defined as a chemical substance which releases one or more OH– ions in aqueous solution.
For example, NaOH, KOH, etc.
- Acid-Base indicator: Natural/synthetic materials which indicate the presence of acid or base in a solution, are called acid base indicator or simply indicator.
- Litmus solution: It is a purple dye which is extracted from lichen, a plant belonging to the division
.
- Phenolphthalein: It is a colourless organic dye in acidic or neutral medium but it changes to pink in basic medium.
- Methyl orange: It is an orange coloured dye and keeps this colour in the neutral medium. In the acidic medium, the colour of the indicator becomes red and in the basic medium, it changes to yellow.
- Red cabbage juice: Its colour remains red in acidic medium but changes to green if the medium is basic or alkaline.
- Turmeric solution: It is a yellow dye and in the acidic as well as neutral medium, its colour remains yellow. In the basic medium the colour changes to reddish brown.
2. Olfactory indicators: These are chemical substances whose odour changes in acidic or basic medium.
For example, onion, vanilla and clove oil.
3. Reaction of acid or base with metal: Metals react with acids to liberate hydrogen gas and form salt.
Acid + Metal → Salt + Hydrogen gas
A few metals like zinc, lead and aluminium react with bases to give off hydrogen.
![]()
4. Reaction of acids with metal hydrogen carbonate and metal carbonates: All metal carbonates and hydrogen carbonates react with acids to give the corresponding salt, carbon dioxide and water.
Metal carbonate + Acid → Salt + H2O + CO2
Metal hydrogen carbonate + Acid → Salt + H2O + CO2
For example,
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
NaHCO3(s) + HCl(ag) → NaCl(aq) + H2O(Z) + CO2(g)
The released CO2 gas turns lime water milky due to formation of CaCO3.
![]()
On passing excess CO2, the milky white precipitate dissolves in water.
![]()
5. Neutralisation reaction: A chemical reaction between an acid and a base to give a salt and water is known as neutralisation reaction. In general neutralisation reaction can be written as
Base + Acid → Salt + Water
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
(i) Reactions of metal oxides with acids
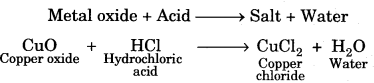
(ii) Reactions of non-metallic oxide with base
Non-metallic oxide + Base→ Salt + Water
CO2 + Ca(OH)2 → CaCO3 + H2O
How strong are acid or base solutions: Any aqueous solution, be it acidic, alkaline or neutral, will have both H+ and OH– ions.
The solution will be acidic or alkaline depending upon which of the two ions is present in larger concentration.
A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. pH scale was given by a Danish chemist Sorensen. The p in pH stands for ‘potenz’ in German meaning ‘power’.
pH should be thought of simply as a number between 0-14 which indicates the acidic or basic nature of a solution. Higher the hydrogen ion concentration, lower is the pH value.
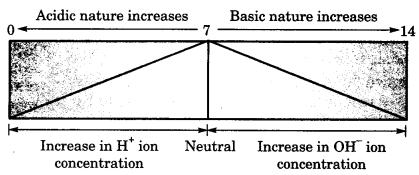
Knowledge +
pH = negative logarithm to the base 10 of the H+ ion concentration.
The concentration is in mol/dm3. pH = – log H+
Remember: Every one fold change in the pH scale brings about a ten-fold change in H+ ion concentration. The strength of acids and bases depends on the concentration of H+ ion and OH–.
If we take two acids HCl and CH3COOH of the same concentration, then they produce different amount of H+. Acids that give rise to more H+ ions are said to be strong acids and acids that give less H+ ions are said to be weak acids.
6. Importance of pH in everyday life:
(i) If pH of rain water is less than 5.6, it is called acid rain. When acid rain flows into the rivers, it lowers the pH of river water. The survival of aquatic life in such rivers become difficult. Acid rain also damage crops and cause a change in pH of the soil.
(ii) pH in our digestive system: Our stomach produces digestive juices/hydrochloric acid (HCl), which helps in the digestion of food without harming the stomach. However, sometimes the stomach produces too much of acid and this causes indigestion, which is accompanied by pain and irritation. To get rid of this pain, people use antacids like magnesium hydroxide. These antacids neutralise the excess acid formed.
(iii) pH change as the cause of tooth decay: Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made up of calcium phosphate is the hardest substance in the body. It does not dissolve in water but is corroded when the pH in the mouth is below 5.5. Using toothpaste, which are generally basic, for cleaning the teeth can neutralise the excess acid and prevent tooth decay.
(iv) Bee-sting leaves an acid which causes pain and irritation. Using a mild base like baking soda on the stung area gives relief. Stinging hair of nettle leaves inject methanoic acid causing a burning pain. A traditional remedy is rubbing the area with the leaf of the dock plant.
(v) Various fluids in our body work within a particular range of pH such as, pH of human blood should be between 7.3 to 7.5.
(vi) For the growth of plants, a particular pH range of soil is essential. Usually neutral soil is best for crops. If the soil is acidic, farmers treat the soil with quick lime or slaked lime.
(vii) The tarnished surface of a copper vessel due to the formation of copper oxide layer (which is basic) can be cleaned by rubbing with lemon (which is acidic).
7. Salts: Salts are generally ionic compounds which are obtained by neutralisation reaction between acids and bases.
Acid + Base→ Salt + Water
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(Z)
In these salts, the cation is derived from base and anion is derived from acid.
Salts are mostly solids with high melting points. They are soluble in water.
Different types of salts:
- Normal salts : NaNO3
- Acidic salts : NaHSO4
- Basic salts : Pb(OH)Cl
- Double salts : FeSO4.(NH4)2.SO4.6H2O
8. Remember: Salts of a strong acid and a strong base are neutral with pH value of 7. On the other hand, salts of strong acid and weak base are acidic with pH values less than 7 and those of strong base and weak acid are basic in nature, with pH value more than 7.
Common Salt (NaCl) [Table salt]
Sodium chloride (NaCl) also called common salt or table salt is the most common/essential part of our diet. It is obtained by neutralisation reaction of sodium hydroxide (NaOH) with hydrochloric acid (HC1).
It is obtained on a large scale from sea water.
Large crystals are often brown due to the presence of impurities. This is called rock salt. Beds of rock salt were formed when seas of by gone ages dried up. Rock salt is mined like coal.
9. Uses of Sodium Chloride
- Sodium chloride is a major ingredient of edible salt.
- It is used as a food preservative.
- Compounds like sodium hydroxide (NaOH), baking soda (NaHCO3) and washing soda are obtained from sodium chloride.
- It is used to melt ice on hill stations and cold countries during heavy snow fall.
10. Sodium Hydroxide (NaOH): Sodium hydroxide (NaOH) is commonly known as caustic soda.
Sodium hydroxide is manufactured by electrolysis of an aqueous solution of sodium chloride (called brine). Chlorine gas is given off at the anode and hydrogen gas at the cathode. Sodium hydroxide solution is formed near the cathode.
2NaCl(aq) + 2H2O(l) → 2NaOH(ag) + Cl2(g) + H2(g)
The process is called the chlor-alkali process because of the products formed chlor for chlorine and alkali for sodium hydroxide.
11. Uses of Sodium Hydroxide
- Sodium hydroxide is used for making soaps and detergents.
- Sodium hydroxide is used for making artificial textile fibres (such as rayon).
- It is used the preparation of soda lime (a mixture of NaOH and CaO).
- It is used as a cleansing agent for machines and metal sheets.
12. Baking Soda (NaHCOs): The chemical name of baking soda is sodium hydrogencarbonate or sodium bicarbonate (NaHCO3). It can be prepared from sodium chloride as
NaCl + H2O + CO2 + NH3→ NH4Cl + NaHCO3
Since it is slightly soluble in water, it can be removed by filtration.
It is a mild non-corrosive base. The following reaction takes place when it is heated during cooking.
![]()
13. Uses of Baking Soda
Being alkaline it is an ingredient in antacids. It neutralises excess acid in the stomach and provides relief.
NaHCO3 + HCl → NaCl + H2O + CO2
- It is used in soda-acid fire extinguisher.
- It is used in making baking powder (a mixture of baking soda and mild edible acid like tartaric acid). When baking powder is heated or mixed in water CO2 gas is released.
NaHCO3 + H+→ CO2 + H2O + Sodium salt of acid
The released CO2 causes breads or cakes to rise making them soft and spongy/fluffy.
14. Bleaching Powder [CaOCl2]: Bleaching powder is calcium oxychloride. It is also known as chloride of lime. Bleaching powder can be prepared by the action of chlorine on dry slaked lime [Ca(OH2)].
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Bleaching powder is a yellowish white solid.
15. Uses of Bleaching Powder
- It is an oxidising agent.
- It is used for disinfecting drinking water to make it free from germs.
- The most important use of bleaching powder is in:
- textile industry for bleaching cotton and linen
- paper industry for bleaching wood pulp
- laundry for bleaching washed clothes.
16. Washing Soda [Na2Og.10H2O]: Sodium carbonate is obtained by heating baking soda. When the sodium carbonate obtained by the above process is recrystallised, we get washing soda.
Na2CO3 + 10H2O → Na2CO3.10H2O
Anhydrous sodium carbonate is called soda ash.
17. Uses of Washing Soda
- It is used in glass, soap and paper industries.
- It is used in the manufacture of borax.
- It is used as a cleaning agent for domestic purposes.
- It is used for removing permanent hardness of water.
18. Plaster of Paris \(\left(\mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}\right)\)
Plaster of Paris is calcium sulphate hemihydrate, it can be obtained by heating gypsum at 373 K.
Plaster of Paris is a white powder and on mixing with water, it changes to gypsum once again giving a hard solid mass.
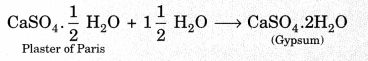
19. Knowledge Booster
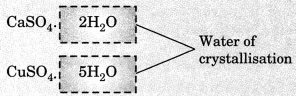
In washing soda, (Na2CO3.10H2O), 10H2O signify water of crystallisation. Water of crystallisation is the fixed number of water molecules present in one formula unit of a salt. Some other examples are
20. Uses of Plaster of Paris
- Plaster of Paris is used by doctors as plaster for supporting fractured bones in the right position.
- It is also used for making toys, materials for decoration and for making surfaces smooth.
Class 10 Science Chapter 2 Notes Important Terms
Alkalies are water soluble bases.
Rock salt is chemically sodium chloride (NaCl).
Antacid is a substance which can neutralise acidity in the stomach.
Neutralisation is the reaction in which an acid reacts with a base to form salt and water.
Bleaching powder is chemically calcium oxychloride (CaOCl2) and is formed by passing chloride gas through dry slaked lime.
Amphoteric compound is a compound that can act both as an acid and a base.
Dilute Acid: Contains only a small amounts of acid and a large amount of water.
Concentrated Acid: A concentrated acid contains a large amount of acid and a small amount of water.