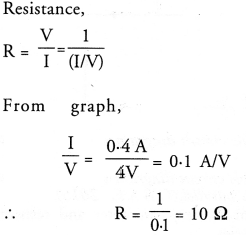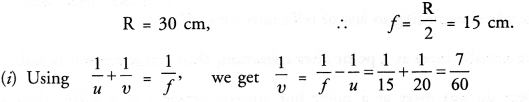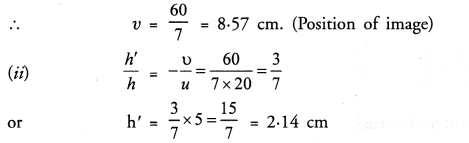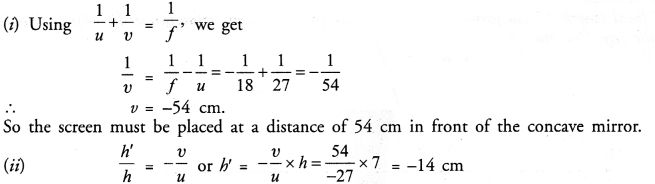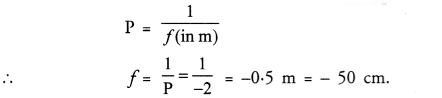NCERT Solutions for Class 10 Science Chapter 16 Management of Natural Resources
These Solutions are part of NCERT Solutions for Class 10 Science. Here we have given NCERT Solutions for Class 10 Science Chapter 16 Management of Natural Resources. LearnInsta.com provides you the Free PDF download of NCERT Solutions for Class 10 Science (Biology) Chapter 16 – Management of Natural Resources solved by Expert Teachers as per NCERT (CBSE) Book guidelines. All Chapter 16 – Management of Natural Resources Exercise Questions with Solutions to help you to revise complete Syllabus and Score More marks.
NCERT Questions
In Text Questions
Question 1.
What changes would you make in your habits to become environment friendly ? (CBSE AI 2009)
Answer:
Three Rs — reduce, recycle and reuse.
- Reduce: It is to reduce consumption by preventing wastage.
- Switching off unnecessary lights, fans and other electrical appliances,
- Repair of leaky taps.
- Reducing food wastage,
- Walking down to nearby market instead of using vehicle.
- Recycle: Separation of recyclable wastes from non-recyclable wastes. The former are taken by rag pickers for sending them to industries involved in recycling, e.g., paper, plastic, metal, glass.
- Reuse: Carry bags, packing material, plastic containers and other reusable articles should not be thrown away if the same are uncontaminated. For example, plastic bottles and jars containing various food items brought from market can be washed and used for storing things in the kitchen.
More Resources
- NCERT Solutions for Class 10 Science
- NCERT Exemplar Solutions for Class 10 Science
- HOTS Questions for Class 10 Science
- Value Based Questions in Science for Class 10
- Previous Year Question Papers for CBSE Class 10 Science
Question 2.
What would be advantages of exploiting resources with short term aims ?
Answer:
- All the commodities for use and comforts for human society will become available in plenty.
- There will be rapid industrialisation and development.
Question 3.
How would these advantages differ from the advantages of using a long term prospective in managing our resources ?
Answer:
|
Short Term Aims |
Long Term Aims |
| 1. All the commodities become available in plenty. | 1. Availability of various commodities is restricted. |
| 2. There is a tendency to manufacture articles of larger size. | 2. There is a tendency to manufacture articles of smaller size. |
| 3. The mentality is of use and throw. | 3. The mentality is reuse, recycle and replace. |
| 4. The resources are likely to get exhausted soon. | 4. The resources are likely to remain available for a long time. |
| 5. The ecology is disturbed and harmed. | 5. It is eco-friendly. |
Question 4.
Why do you think there should be equitable distribution of resources ? What forces would be working against an equitable distribution of our resources ?
Answer:
Need for Equitable Distribution. Resources are living and non-living components of nature which are drawn upon to provide food, fodder, shelter, water, energy, articles of daily use and comforts. Every human being has the fundamental right to obtain and use the same. This is possible only when there is equitable distribution of resources.
Forces Against Equitable Distribution,
- Restricted availability of resources,
- Excessive consumption by the rich,
- Profit motto by the persons exploiting resources.
Question 5.
Why should we conserve forests and wildlife ?
Answer:
There are economic and ecological reasons to conserve forests and wildlife.
- Economic Reasons:
- Forests are a source of shelter, food and fodder to people living in and around forests,
- They provide firewood and timber,
- A number of industrial raw materials are obtained from forests, e.g., tannins, dyes, resins, perfumes, wood for paper,
- Several medicines are got from forest plants and animals,
- Wildlife is a gene bank for improvement of domesticated plants and animals,
- All plants and animals have not yet been explored for their potential uses.
- Ecological Reasons:
- They regulate climate,
- Forests help in retaining rain water and its storage in aquifers from where water becomes available to springs and streams,
- Forests control soil erosion and prevent the occurrence of floods,
- Wildlife is essential for maintaining the forests.
- CO2-O2 balance is maintained.
Question 6.
Suggest some approaches towards the conservation of forests.
Answer:
Conservation is protection, augmentation and scientific management of a resource so as to maintain it at its optimum level while providing sustainable benefits for the present as well as future generations. Some approaches for conservation of forests are as follows :
- Afforestation and Reforestation. Afforestation is developing forest over an area where none existed earlier. Reforestation is developing forest cover which has been cleared during exploitation. Degraded forests are also mended to bring them back to health. All this is done by state forest department either by themselves or with the help of joint forest management committees.
- Separation of Commercial Forestry. Useful plants required for meeting commercial needs should be planted separately so that there is no undue pressure on natural forests. Social forestry is growing multipurpose plants on village common lands for meeting requirement of fodder, firewood and small timber. Production plantation is growing industry required plants on wastelands.
- Controlled grazing.
- Prevention of scraping and litter removal.
- Practising block cutting or cutting of forest equal to its regeneration capacity.
- Building of national parks, sanctuaries and biosphere reserves.
Question 7.
Find out about traditional systems of water harvesting management in your region.
Answer:
Ponds, pools, tanks, lakes, ditches and wetlands. They get filled up during rain. Larger reservoirs are perennial. The smaller ones dry up after a few months. However, both help in recharge of ground water.
Question 8.
Compare the above system with the probable system in hilly/mountainous areas or plains or plateau regions.
Answer:
Hilly/Mountainous Areas. Water is impounded on the slopes, at the base of slope, on the border of ravine or melting snow into tanks called Kulhs, Khatris, Kuls (Himachal Pardesh, J and K), Zabo (Nagaland) and Zings (Ladakh). Water from tanks is used for different purposes—drinking, washing, animals, irrigation.
Plains,
- Roof top harvesting of rain water has been in practice since ages in Rajasthan. The collected water is stored below the ground level for later use.
- Other methods of water harvesting are specially constructed tanks, Kuis, jhaslars, booris or bers, paar, vivdas, khadins and nadis (Rajasthan), ponds in Kandi belts (Jammu), bhandaras and tals (Maharashtra), bundhis (MP and UP), ahars and pynes (Bihar).
Plateau. Water is collected in series of tanks through impounding rain water, building check dams and channels from flowing water. They are called eris (Tamil Nadu), surrangams (Kerala) and kattas (Karnataka).
Question 9.
Find out the source of water in your region!locality. Is water from this source available to all people living in that area ?
Answer:
Water is supplied by municipal corporation through pipes. It is obtained from river/canal (if the same happens to be nearby). In many cases piped water is pumped out from ground by tube wells and stored in raised water tanks.
Municipal water supply is not available to all the residents. Many newly built colonies, slums and unauthorised localities are often without the piped water.
NCERT Chapter End Exercises
Question 1.
What changes would you suggest in your home in order to be environment friendly ?
(CBSE AI 2009, CCE 2012, 2014)
Answer:
- Judicious use of electricity by switching off lights and electrical appliances not required,
- Replacement of incandescent bulbs with fluorescent, compact fluorescent ones and LED bulbs.
- Replacement of electricity or gas operated geysers with solar water heaters,
- Replacement of electricity generating sets with solar light,
- Having more natural light and ventilation with design supporting warming during winters and cooling during summer,
- Reducing wastage of water, food and other articles.
- Separation of recyclable waste from non-cyclable waste prior to disposal.
- Increasing reuse of containers,
- Using cloth bags instead of polythene, plastic or paper bags.
Question 2.
Can you suggest some changes in your school which would make it environment friendly ?
Answer:
Yes.
- Rainwater harvesting (RWH) by draining roof top rain for underground seepage,
- Plantation of trees and shrubs along the boundary for protection against noise and air pollution. Plants will not only provide more oxygen during the school hours but also have a moderating effect on climate.
- Prevention of wastage of electricity and water,
- Having compost pits for conversion of plant refuse and other biodegradable wastes into useful resource,
- Increasing use of solar energy,
- Activating ecoclub.
Question 3.
There are four main stakeholders when it comes to forests and wildlife. Which among these should have the authority to decide the management offorest produce ? Why do you think so (CBSE Foreign 2010, CCE 2011)
Answer:
The four stakeholders in forests and wildlife are
- People living in and around forests who obtain most of their requirements from forests,
- Forest department which owns the forests.
- Industrialists who obtain raw materials for their industries,
- Wildlife and nature enthusiasts. Among them the persons living in and around the forests have traditional knowledge of how best the forests can be managed so that a sustainable yield can be obtained indefinitely. Therefore, authority for management of forest produce should be handed over to them. However, the forest department must monitor the same so that there is no excessive exploitation.
Question 4.
How can you as an individual contribute or make a difference to the management of
(a) Forests and wildlife
(b) Water resources
(c) Coal and petroleum ?
Answer:
(a) Forests and Wildlife:
- I will stress on sustainable use of produce from forests and wildlife,
- The area under forest cover has to be increased to control global warming.
- Form more joint forest management committees,
- Separate commercial forestry from natural forestry.
(b) Water Resources. My major stress will be
- Recharging of ground water through rain water harvesting, protection to wetlands and digging sponge pits or wells in the beds of rivulets,
- Reducing spoilage of water in agriculture by helping formers to switch over to sprinkler system of irrigation, changing conventional method of rice cultivation with system of rice intensification (SRI),
- Judicious use of water for domestic and industrial purposes.
(c) Coal and Petroleum:
- Higher level of purification to reduce pollution,
- Increased use of solar energy,
- More investment to commercialise hydrogen powered transport system.
Question 5.
What can you, as an individual, do to reduce your consumption of various natural resources ?
Answer:
Follow the principle of three Rs, i.e, reduce, recycle, reuse.
Question 6.
List five things you have done over the last one week to
(a) Conserve our natural resources
(b) Increase the pressure on our natural resources ?
Answer:
(a) Conservation of Natural Resources
- Reduced consumption of electricity by switching off unwanted fans and bulbs,
- Reduced consumption of water by closing the tap to prevent overflow of water in the bucket during taking bath,
- Used refills instead of purchasing new pens,
- I did not waste food,
- I have started going to school by school bus instead of using car.
(b) Increase Pressure on Natural Resources
- While reading at night on bed, I fell asleep and forgot to switch off the lights,
- While brushing my teeth and later taking bath, I did not close the tap, so a lot of water was wasted,
- I left the ball pen uncapped, so the ink dried up. I had to throw three ball pens last week,
- I am in the habit of using loose sheets for doing rough work. They are then thrown away,
- I bring more food in my tiffin than I eat. Daily I throw a lot of food in the dust bin.
Question 7.
On the basis of environmental issues you studied, what changes would you incorporate in your life style in a move towards a sustainable use of our resources ?
Answer:
1. Follow the principle of three Rs — reduce, recycle and reuse.
2. Undertake tree plantation twice a year during days of Van Mahotsava.
3. Where possible, use of public transport, school, bus and car pools.
4. Use of solar energy for as many purposes as possible.
Selection Type Questions
Alternate Response Type Questions
(True/False, Right(√)/Wrong (x), Yes/No)
Question 1.
Presence of coliform bacteria in a water body indicates contamination by industrial effluents.
Question 2.
An environmentally friendly decision is reuse jam and pickle bottles.
Question 3.
Forests are reservoirs of wildlife.
Question 4.
Chipko andolan originated in Haridwar during 1980s.
Question 5.
Check dams are built along seasonal flooded gullies for water harvesting.
Question 6.
Coal and petroleum are non-renewable inexhaustible resource.
Question 7.
An important protective function of forests is reduction of atmospheric pollution.
Question 8.
Forest department has been able to maintain biodiversity by growing pine, Teak and Eucalyptus on large tracts.
Matching Type Questions
Question 9.
Match the columns I and II (single matching) :
|
Column I |
Column II |
|
(a) Desertification (b) Khejri (c) Khadin (d) Carbon monoxide |
(i) Amrita Devi Bishnoi (ii) Incomplete combustion (iii) Deforestation (iv) Water harvesting |
Question 10.
Match the contents of columns I, II and III (double matching) :
|
Column I |
Column II |
Column III |
|
(a) Flyash (b) Kulhs (c) Van Mahotsav (d) Water pollution |
(i) Coliform count
(ii) Coal (iii) Water harvesting (iv) Afforestation |
p. Himachal Pradesh q. BOD r. Toxic chemicals s. February, July |
Question 11.
Mark the resource as I (inexhaustible), R (renewable) and N (non-renewable) (key or check list items):

Question 12.
Match stimulus with appropriate response.
|
Water Harvesting |
Karnataka A | Tamil Nadu B | Kerala C |
|
(i) Surrangams (ii) Kattas (iii) Eris |
Fill in the Blanks
Question 13. Forests are ………….. hot spots.
Question 14. Amrita Devi Bishnoi sacrificed her life and that of 363 others in for ……………….. protection of …………….. trees.
Question 15. In blood carbon monoxide forms …………… that is unable to transport oxygen.
Question 16. Ganga runs its course of 2500 km from …………….. in Bay of Bengal.
Question 17. To save energy and prevent warming, I use ……………… instead of incandescent bulbs.
Answers:
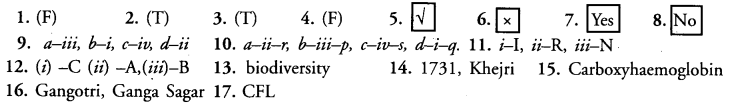
NCERT Solutions for Class 10 Science Chapter 16 Management of Natural Resources
Hope given NCERT Solutions for Class 10 Science Chapter 16 are helpful to complete your science homework.
If you have any doubts, please comment below. Learn Insta try to provide online science tutoring for you.