Students can access the CBSE Sample Papers for Class 10 Science with Solutions and marking scheme Set 3 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Science Set 3 with Solutions
Time: 3 Hours
Maximum Marks: 80
General Instructions:
(i) The question paper comprises four sections A, B, C and D. There are 36 questions in the question paper. All questions are compulsory.
(ii) Section-A – question no. 1 to 20 – all questions and parts there of are of one mark each.
These questions contain multiple choice questions (MCQs), very short answer questions and assertion – reason type questions. Answers to these should be given in one word or one sentence.
(iii) Section-B – question no. 21 to 26 are short answer type questions, carrying 2 marks each. Answers to these questions should in the range of 30 to 50 words.
(iv) Section-C – question no. 27 to 33 are short answer type questions, carrying 3 marks each.
Answers to these questions should in the range of 50 to 80 words. :
(v) Section—D – question no. – 34 to 36 are long answer type questions carrying 5 marks each. Answer to these questions should be in the range of 80 to 120 words.
(vi) There is no overall choice. However, internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
(vii) Wherever necessary, neat and properly labelled diagrams should be drawn.
Section – A
Question 1.
Name a cyclic unsaturated carbon compound.
Answer:
Cyclopentene / Cyclohexene-formula or structure (or any other).
Question 2.
The change in magnetic field lines in a coil is the cause of induced electric current in it. Name the underlying phenomenon.
Answer:
Electromagnetic Induction
Question 3.
Why do we store silver chloride in dark coloured bottles?
Answer:
It is because it undergoes photochemical decomposition reaction in the presence of sunlight.
Question 4.
How many male gametes are produced by pollen grains?
Answer:
Two
Question 5.
Two beakers with chemicals are shown below. Name the beaker which will show exothermic reaction and the one which will be endothermic in nature.
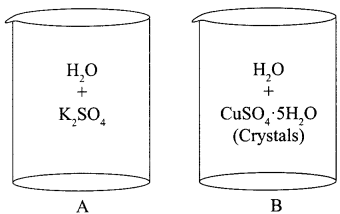
Answer:
Exothermic : Beaker A
Endothermic : Beaker B
Question 6.
What will happen, if a solution of sodium hydrogen carbonate is heated? Give the equation of the reaction involved.
OR
How chloride of lime differs chemically from calcium chloride?
Answer:
Sodium carbonate, carbon dioxide and water will be formed.
\(2 \mathrm{NaHCO}_{3}(\mathrm{~s}) \stackrel{\text { Heat }}{\longrightarrow} \mathrm{Na}_{2} \mathrm{CO}_{3}(\mathrm{~s})+\mathrm{CO}_{2}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{O} \text { ( } \mathrm{l}\)
Question 11.
What will happen to a plant if its xylem is removed?
OR
Why is it advisable to breathe through nose?
Answer:
If xylem is removed from a plant, transport of water and nutrients will stop and plant will die.
OR
There are fine hair and mucus gland in the inner lining of nose which can filter the incoming air from germs and dust. Moreover, the air attains the optimum temperature before reaching the lungs.
Question 12.
An element ‘X’ on reacting with oxygen forms an oxide X2O. This oxide dissolves in water and turns blue litmus red. State whether ‘X’ is metal or non-metal.
Answer:
Non-metal
Question 13.
What is the speed of blue light travelling in vacuum?
Answer:
The speed of blue light is same as that of light i.e. 3 x 108 m s–1.
Assertion (A) and Reason (R)
For question numbers 14,15 and 16, two statements are given- one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (i), (iii) and (iv) as given below:
(i) Both A and R are true, and R is correct explanation of the assertion.
(ii) Both A and R are true, but R is not the correct explanation of the assertion.
(iii) A is true, but R is false.
(iv) A is false, but R is true.
Question 14.
A. The element carbon forms the basic structural framework of more compounds than any other element.
R. The carbon-carbon bond is ionic.
Answer:
(iii)
Question 15.
A. Pollination is different from fertilisation.
R. The process of transfer of pollens from anther to stigma is called pollination which takes place in plants.
Answer:
(iv)
Question 16.
A. A 200 W bulb glows with more brightness than 100 W bulb.
R. 100 W bulb has more resistance than 200 W bulb.
OR
A. When two resistances of 4Ω are connected in series, total resistance is 8Ω.
B. When two resistances of 4Ω are connected in parallel total resistance is 2Ω.
Answer:
(i) OR (ii)
Answer Q. No 17 – 20 contain five sub-parts each. You are expected to answer any four subparts in these questions.
Question 17.
Read the following and answer any four questions from 17 (i) to 17 (v) (4 × 1 = 4)
The growing size of the human population is a cause of concern for all people. The rate of birth and death in a given population will determine its size. Reproduction is the process by which organisms increase their population. The process of sexual maturation for reproduction is gradual and takes place while general body growth is still going on. Some degree of sexual
chemicals are not biodegradable, they get accumulated progressively at each trophic level. The maximum concentration of these chemicals gets accumulated in our bodies and greatly affects the health of our mind and body.
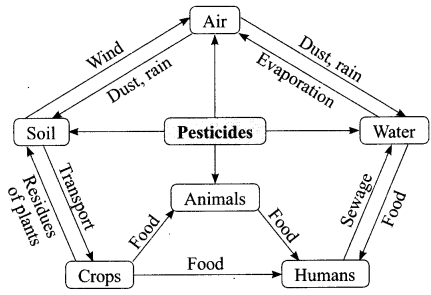
(i) Why is the maximum concentration of pesticides found in human beings?
(a) Humans are at the top level in any food chain
(b) Major part of crops cultivated using pesticides are consumed by humans
(c) Pesticides are stored in different organs of humans
(d) None of these
Answer:
(a) Humans are at the top level in any food chain
(ii) Which method could be applied to reduce our intake of pesticides through food to some extent.
(a) Washing crops thoroughly
(b) Organic fanning
(c) Use of bio-pesticides
(d) All of these
Answer:
(d) All of these
(iii) Various steps in a food chain represent:
(a) Food web
(b) Trophic level
(c) Ecosystem
(d) Biomagnification
Answer:
(b) Trophic level
(iv) With regard to various food chains operating in an ecosystem, man is a:
(a) Consumer
(b) Producer
(c) Producer and consumer
(d) Producer and decomposer
Answer:
(a) Consumer
(v) Food web is constituted by
(a) relationship between the organisms and the environment.
(b) relationship between plants and animals.
(c) various interlinked food chains in an ecosystem.
(d) relationship between animals and environment
Answer:
(c) various interlinked food chains in an ecosystem.
Question 19.
Read the following and answer any four questions from 19 (i) to 19 (v) (4 × 1 = 4)
Electrical resistivity and its inverse, electrical conductivity are fundamental properties of a material that quantifies how strongly it resists or conducts electric current. A low resistivity indicates a material that readily allows electric current to flow.
Resistance is defined as the ratio of potential difference across a conductor to the current passing through it. Resistance is the property of a conductor to resist the flow of charges through it.
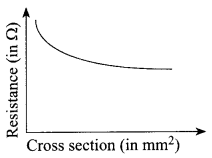
Both resistance and resistivity describe how difficult it is to G make electrical current flow through a material, but unlike w resistance, resistivity is an intrinsic property. This means that g all pure copper wires irrespective of their shape and size, have the same resistivity, but a long, thin copper wire has a much larger resistance than a thick, short copper wire. Every material has its own characteristic resistivity. For example, rubber has a far larger resistivity than copper. The resistivity of metals also varies with temperature.
The resistance of almost all alloys increases with increase in temperature but the rate of change of resistance is less than that of metals. In fact, the resistance of certain alloys such as Manganin, Eureka and Constantan show practically no change in resistance to a considerable range of temperature. ‘
(i) What happens to the resistivity of a wire if it is stretched?
(a) It will increase
(b) It will decrease
(c) First increases then decreases
(d) Remains the same
Answer:
(d) Remains the same
(ii) Though silver is a good conductor, why it is not an ideal choice for transmission of electricity?
1. It is expensive
2. It oxidizes and tarnishes when it comes in contact with air
3. Its resistivity decreases with increase in temperature
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Only 3
Answer:
(c) Both 1 and 2
(iii) The area of cross section of a wire becomes half when its length is stretched to double. How the resistance of the wire is affected in the new condition?
(a) Resistance of the wire remains unchanged
(b) Resistance of the wire decreases to half
(c) Resistance of the wire increases to double
(d) Resistance of the wire increases four times
Answer:
(d) Volume of the wire remains the same before and after stretching. Assuming wire to be cylindrical in shows them volume = Area of base x height/length
Due to stretching if length is increased, area will decrease.
R = ρl/A
New length = 2l
New area = A/2
Resistance increases four times.
(iv) If there are two wires, W1 and W2 of same material and same length but have radius r and 2r respectively, then which wire will have more resistance?
(a) Wire W1 has more resistance as compare to W2
(b) Wire W2 has more resistance as compare to W1
(c) Both the wires have same resistance
(d) Wire W2 has three times resistance than wire W1
Answer:
(a) Wire W1 has more resistance as compare to W2
(v) Ajay made the following conclusions regarding resistance of metals/alloy. Which one would you disagree with?
(a) The resistivity of a metal decreases with temperature
(b) A thick copper wire has less resistance than a thin copper wire of the same length
(c) Alloys are often used in the manufacture of standard resistors
(d) Low resistivity means the material easily allow the flow of electric current
Answer:
(a) The resistivity of a metal decreases with temperature
Question 20.
Read the following and answer any four questions from 20 (i) to 20 (v) (4 × 1 = 4)
The following graph shows the volume of hydrogen collected in four different experiments when dil. hydrochloric acid was reacted with zinc, copper, magnesium and iron metal.
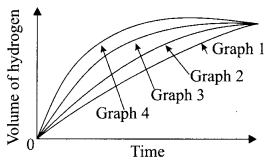
(i) Name the graph that produces hydrogen gas faster.
(a) Graph 1
(b) Graph 2
(c) Graph 3
(d) Graph 4
Answer:
(d) Graph 4
(ii) Name the metal used in experiment that gave the result for graph-4.
(a) Mg
(b) Zn
(c) Fe
(d) Cu
Answer:
(a) Mg
(iii) Ways to speed up the slow reaction are
1. heating the reactants
2. cooling the reactants
3. using more diluted acid
4. using more concentrated acid
(a) 1 only
(b) 1 and 4
(c) 1 and 3
(d) 2 and 4
Answer:
(b) 1 and 4
(iv) The amount of zinc metal used for the above reaction is same but the concentration of acid is changed, what would be the effect on the rate of reaction?
(a) no change
(b) the speed will increase
(c) the speed will decrease
(d) the information is incomplete
Answer:
(d) the information is incomplete
(v) Which of the following metal liberates hydrogen gas on reaction with dilute HNO3.
(a) Zn
(b) Cu
(c) Mg
(d) Fe
Answer:
(c) Mg
Section – B
Question 21.
The period 3 oxides are given below.
[Na2O, MgO] [Al2O3]……………………. [SO2, Cl2O]
A B C
State 2 characters of these oxides grouped as A & C
Answer:
Oxide A: Na2O, MgO are basic in nature and ionic Oxide C : SO2, Cl2O are acidic in nature and covalent
Question 22.
Name two human organs that perform dual function and explain their functions to justify their dual nature.
Answer:
Two human organs with dual nature are pancreas and gonads. Pancreas secretes insulin hormone and digestive enzymes like trypsin ( pancreatic enzyme) similarly human gonads like testis and ovaries secretes the gametes like sperms and ovum respectively along with the sex hormones.
Question 23.
Why are small number of surviving tigers a cause of worry from genetics point of view?
Answer:
(i) Tigers may get extinct in near future due to natural calamities, lack of available food and due to their small number.
(ii) Cross breeding may be less possible. Variations will not occur, which is essential for the survival.
(iii) Adaptation due to changing environment, like cutting of forests, global warming becomes difficult.
(iv) Protection from enemies is easy if they are more in number.
Question 24.
An object is placed at a distance of 15 cm from a concave lens of focal length 30 cm. List four characteristics (nature, position, etc.) of the image formed by the lens.
Answer:
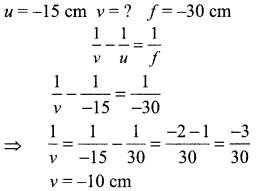
The image will be formed at 10 cm from ‘O’ on the same side as the object. It will be virtual, erect and diminished.
Question 25.
Write chemical equation for the reaction when
(i) Steam acts on red hot iron.
(ii) Zinc is added to iron(II) sulphate solution.
OR
State two reasons for the following facts:
(i) Sulphur is a non-metal.
(ii) Magnesium is a metal.
One of the reason must be supported with a chemical equation.
Answer:
(i) \(3 \mathrm{Fe}(s)+4 \mathrm{H}_{2} \mathrm{O}(g) \underset{\text { steam }}{\longrightarrow} \mathrm{Fe}_{3} \mathrm{O}_{4}(s)+4 \mathrm{H}_{2}(g)\)
(ii) Zn(s) + FeSO4(aq) → ZnSO4(aq) + Fe(s)
OR
(i) Sulphur is a poor conductor of electricity.
It reacts with oxygen to form SO2 which forms acidic solution in water.
S + O2 → S02
SO2 + H2O →H2SO3
(ii) Magnesium is a metal because it is a good conductor of electricity.
It forms basic oxide with oxygen
\(2 \mathrm{Mg}+\mathrm{O}_{2} \stackrel{\text { Burning }}{\longrightarrow} 2 \mathrm{MgO}\)
\(\mathrm{MgO}+\underset{(\mathrm{Hot})}{\mathrm{H}_{2} \mathrm{O}} \rightarrow \underset{(\text { Base })}{\mathrm{Mg}(\mathrm{OH})_{2}}\)
Question 26.
Study the following circuit and answer the questions that follows:
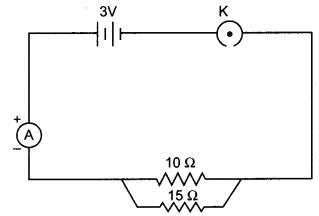
(i) How much current is flowing through
(a) 10 Q and
(b) 15 Q resistor?
(ii) What is the ammeter reading?
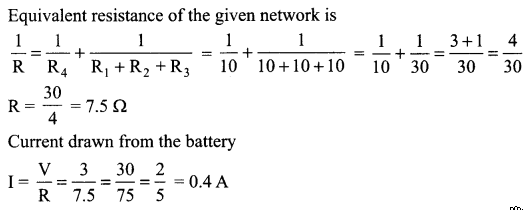
OR
Find the current drawn from the battery by the network of four resistors shown in the figure.
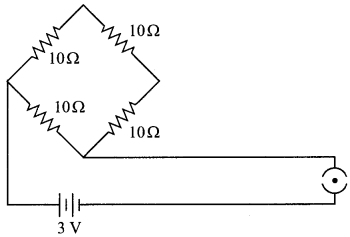
Answer:
(i) Current through
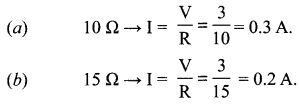
Question 27.
1 g of copper powder was taken in a China dish and heated. What change takes place on heating? When hydrogen gas is passed over this heated substance, a visible change is seen in it. Give the chemical equations of reactions, the name and the color of the products formed in each case.
Answer:
A black colour is formed on the surface
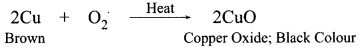
Original/brown colour is restored
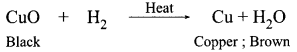
Question 28.
List the important products of the Chlor-alkali process. Write one important use of each.
OR
How is washing soda prepared from sodium carbonate? Give its chemical equation. State the type of this salt. Name the type of hardness of water which can be removed by it?
Answer:
Products: Hydrogen, Chlorine, Sodium hydroxide Uses:
Hydrogen: In the production of margarine/ammonia/as a fuel
Chlorine: Water treatment / swimming pools / production of PVC / Disinfectants / CFCs / Pesticides
Sodium hydroxide: For degreasing metal surface / in making soaps and detergents / paper making / artificial fibres.
OR
1. By recrystallisation of sodium carbonate
2. Na2CO3 + 10H2O → Na2CO3.10H2O
3. Basic Salt
4. Permanent hardness
Question 29.
Study the following cross showing self pollination in F1, fill in the blank and answer the question that follow:
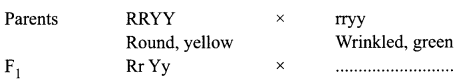
What are the combinations of character in the F2 progeny? What are their ratios?
Answer:
F1 progeny is Rr Yy-Round, Yellow
Combinations of character in the F2 progeny are:
- Round yellow — 9 (Both dominant traits)
- Round green — 3 (One recessive, one dominant)
- Wrinkled yellow — 3 (One dominant, one recessive)
- Wrinkled green — 1 (both recessive traits)
The ratio is 9 : 3 : 3 : 1
Question 30.
Define the term pollination. Differentiate between self pollination and cross pollination. What is the significance of pollination?
Answer:
Pollination is the transfer of pollen from another to stigma.
| Self Pollination | Cross Pollination |
| Transfer of pollen in the same flower. | Transfer of pollen from one flower to another. |
• Pollination leads to fertilization resulting in the formation of zygote.
Question 31.
Why is Tyndall effect shown by colloidal particles? State four instances of observing the Tyndall effect.
Answer:
Because of the scattering of light.
Instances:
- When a fine beam of light enters a smoke-filled dark room through a small hole.
- When sunlight passes through a canopy of dense forest in foggy/ misty conditions.
- Blue colour of sky.
- Red colour of the sun during sunrise or sunset.
Question 32.
A V-I graph for a nichrome wire is given below. What do you infer from this graph? Draw a labelled circuit diagram to obtain such a graph.
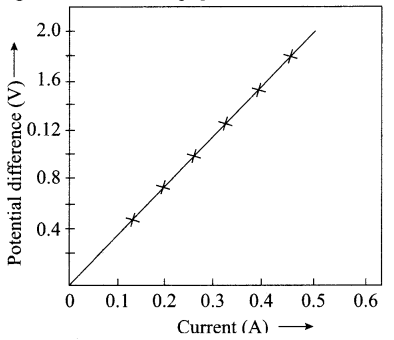
Answer:
V α I or Potential difference is directly proportional to current
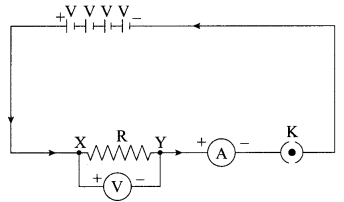
Note: If circuit diagram is correct but labelling of ammeter and voltmeter are incorrect, deduct 1 mark.
Question 33.
(i) Write the mathematical expression for Joule’s law of heating.
(ii) Compute the heat generated while transferring 96000 coulombs of charge in two hours through a potential difference of 40 V.
Answer:
(i) H = I2Ri
(ii) H = V.I.t = V.Q
Given: V = 40 volts, Q = 96000 C
H = 40 V x 96000 C
= 3.84 x 106 J
Section – D
Question 34.
The position of certain elements in the Modem Periodic Table are shown below.
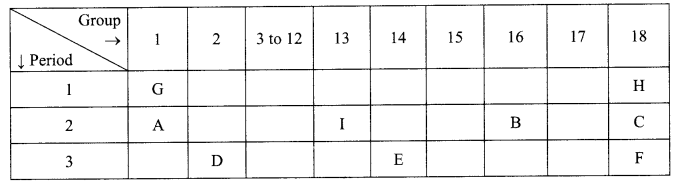
Using the above table answer the following questions giving reasons in each case:
(i) Which element will form only covalent compounds?
(ii) Which element is a non-metal with valency 2?
(iii) Which element is a metal with valency 2?
(iv) Out of H, C and F which has largest atomic size?
(v) To which family does H, C and F belong?
OR
Define atomic size. Give its unit of measurement. In the modem periodic table what trend is observed in the atomic radius in a group and a period and why is it so?
Answer:
(i) E, it has 4 valence electrons.
(ii) B, it needs only 2 electrons to attain stable configuration.
(iii) D, it loses two electrons to attain stable configuration.
(iv) F, it has the largest size since size increases down the group.
(v) Noble gases, outermost shell is complete.
OR
- Atomic size is the distance between the centre of the nucleus and the outermost shell of an isolated atom.
- Picometer /pm
- Trends in Atomic radius
In a group: increases down the group; due to addition of a new shell.
In a period: atomic radius decreases from left to right; due to increase in pulling power of nucleus / due to addition of electrons in the same shell.
Question 35.
(i) What is the law of dominance of traits? Explain with an example.
(ii) Why are the traits acquired during the life time of an individual not inherited? Explain.
Answer:
(i) Law of dominance of traits: In a cross between a pair of contrasting characters, only one parental character will be expressed in F1 generation which is called dominant trait and the other is called a recessive trait.
For example – in pea plants.
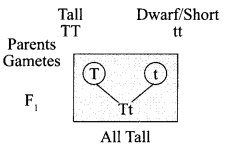
All plants in F1 generation were tall proving that the gene for tallness is dominant over the gene for dwarfness/short, which is not able to express itself in the presence of dominant trait.
(ii) Traits acquired by an organism during its lifetime are known as aquired traits. These traits are not inherited because they occur in somatic cells only/do not cause any change in the DNA of the germ cells.
Question 36.
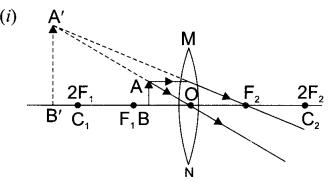
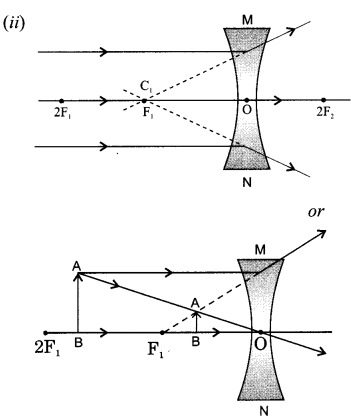
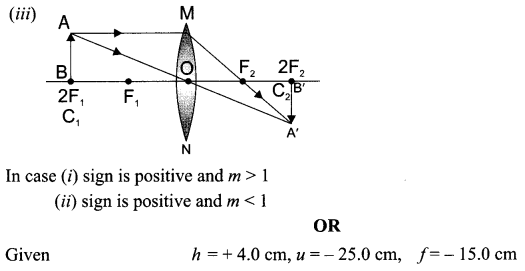
Draw a ray diagram in each of the following cases to show the formation of image, when the object is placed:
(i) between optical centre and principal focus of a convex lens.
(ii) anywhere in front of a concave lens.
(iii) at 2F of convex lens.
State the signs and values of magnifications in the above mentioned cases (i) and (ii).
OR
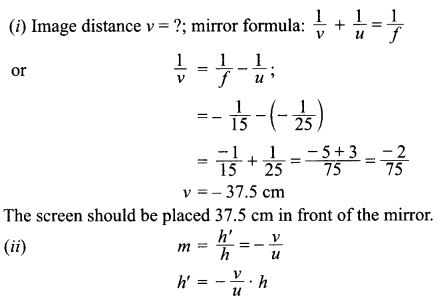
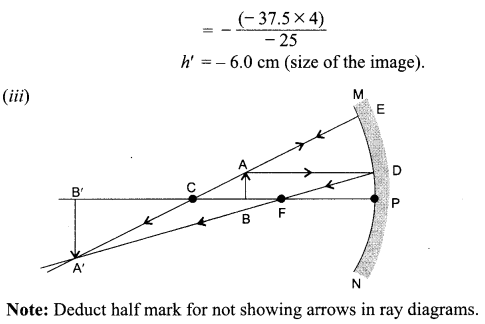
An object 4.0 cm in size, is placed 25.0 cm in front of a concave mirror of focal length 15.0 cm.
(i) At what distance from the mirror should a screen be placed in order to obtain a sharp image?
(ii) Find the size of the image.
(iii) Draw a ray diagram to show the formation of image in this case.
Answer: