Students can access the CBSE Sample Papers for Class 10 Science with Solutions and marking scheme Term 2 Set 1 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Science Term 2 Set 1 with Solutions
Time allowed: 2 Hours
Maximum Marks: 40
General Instructions:
- All questions are compulsory.
- The question paper has three sections and 15 questions. ALL questions are compulsory.
- Section-A has 7 questions of 2 marks each; Section-B has 6 questions of 3 marks each, and Section-C has 2 case based questions of 4 marks each.
- Internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
Section – A
Question 1.
Genetics is an interesting fieLd of science. This branch of science finds wide application in various sectors such as crime investigation, forensic, archaeology, etc.
(A) What for did Mendel used the term factors and what are these factors called now? (1)
Answer:
Mendel used the term factors for ‘genes’.
(B) What are genes? Where are the genes located? (1)
Answer:
Gene is the unit of inheritance. It is a part of the chromosome which controls the appearance of a set of hereditary character. Genes are located in the chromosome.
Question 2.
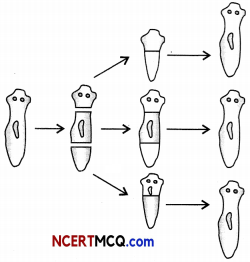
The figure shows a mode of reproduction.
Based on the figure, identify the type of reproduction and mode of reproduction Explain.
OR
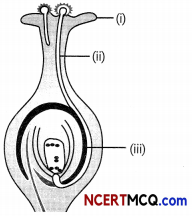
Identify the parts of a carpel and write the function of each part.
Answer:
The type of reproduction is asexual reproduction and the mode of reproduction is regeneration.
Regeneration in Planaria: Planaria can be cut into any number of pieces and each piece grows into a complete organism. This is known as regeneration. Regeneration is carried out bu specialised cells. These cells proliferate and make a large numbers of cells. From this mass of cells, different cells undergo changes to become various cell types and tissues. These changes take place in an organised sequence referred to as development.
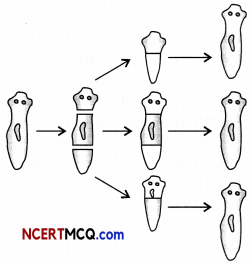
Regeneration in Planaria
OR
(i) Sigma, (ii) Style, (iii) Ovary
Functions of these part are:
Stigma: It is the topmost part of the carpet and receives the pollen grains from the anther of the stomen during poWnation. It is sticky so that the pollen sticks to it.
Style: It is a tube Like structure that connects ovary and stigma. It is a passage for the growth of pollen tube.
Ovary: It is a swollen part at the bottom of the carpel The ovary contains ovules and ovules contain the female gametes (eggs).
![]()
Question 3.
(A) Consider the food chain: grass → deer lion. What will happen if lions are removed from the given food chain? (1)
Answer:
Removal of Lions from the food chain wilt increase number of deer in the food chain. The grass (producer) is eaten up by the deer. Hence, producers and the land will become arid.
Related Theory:
In this food chain grass is a producer, deer is herbivore and tiger is carnivore.
(B) Why are some substances biodegradable and some non-biodegradable? (1)
Answer:
In our environment micro-organisms such as bacteria and fungi secrete enzymes. These enzymes degrade organic compounds, present in the dead remains of plants and animals and their waste products into simpler harmless substances. These wastes are termed as biodegradable. On the other hand, these enzymes cannot degrade certain categories of waste like plastics, glass etc. These waste persist in the environment and are termed as non- biodegradable.
Question 4.
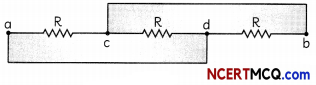
What is the resistance between ‘a’ and ‘b’ in the network shown in the figure?
OR
In the circuit diagram given here, calculate the total effective resistance.
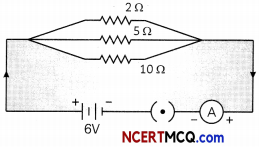
Answer:
On careful observation it can be seen that the combination is parallel as shown below.
OR
It can be seen that the resistances are in parallel. Hence net resistance is:
\(\frac{1}{R}=\left(\frac{1}{2}+\frac{1}{5}+\frac{1}{10}\right)^{-1}\)
\(\frac{1}{R}=\left(\frac{5}{10}+\frac{2}{10}+\frac{1}{10}\right)^{-1}\)
= \(\left(\frac{8}{10}\right)^{-1}=\frac{10}{8}\)
= 1.25 Ω
Question 5.
The structural formula of five compounds are given here:
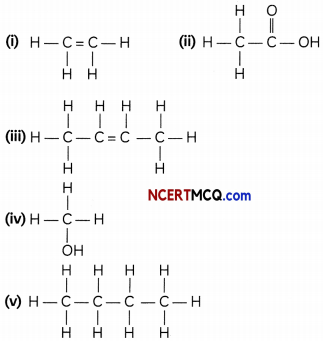
(A) Which two compounds belong to the same homologous series? (1)
Answer:
Compound (i) and (iii) belong to the same homologous series. They both belong to alkene family as they satisfy the general formula of CnH2n
(B) Which compound belongs to the same homologous series as ethanol? (1)
Answer:
Compound (iv) belongs to the same family as that of ethanol as they both have the functional group —OH, alcohol.
![]()
Question 6.
(A) Menstruation is a monthly cycle in females. However, if fertilisation happens, menstruation will not occur. What changes can be expected if fertilisation does not occur? (1)
Answer:
The uterus prepares itself during every ovulation cycle in anticipation of a possible pregnancy. The lining of the uterus thickens so that it can give support to the developing embryo. When fertilisation does not occur, this lining disintegrates because it is no longer required. The fragments of the lining are shed along with blood and the discarded egg; through the vagina. The discharge of the discarded tissue is called menstruation.
(B) Sexually Transmitted Diseases are a real threat to the mankind. However, there are ways to prevent STDs. Can you give one example of STD caused by bacteria and virus each and suggest ways to prevent it. (1)
Answer:
Gonorrhoea is caused by bacteria and AIDS is caused by virus. These diseases can be prevented by responsible sexual behaviour such as use of condom during sexual intercourse, etc.
Question 7.
Using the part of the periodic table given below answer the questions that follows:
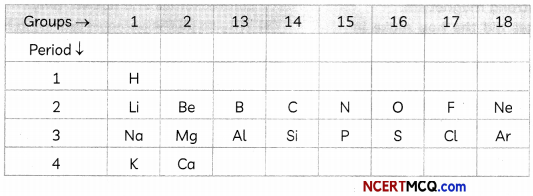
(A) (i) Na has physical and chemical properties similar to which elements). (½)
(ii) Write the electronic configuration of N and P. Which one of these will be more electronegative and why? (1)
(B) Write the formulae of chlorides of EKa- silicon and Eka-aluminium, the elements predicted by Mendeleev. (½)
OR
Using the part of a periodic table, answer the following questions:
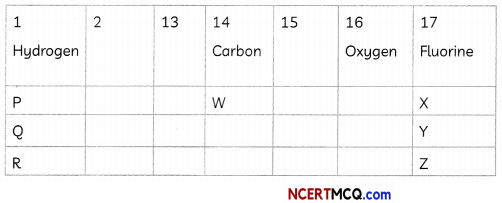
(A) Atomic number of Carbon is 6. What would be the atomic number of Fluorine? (1)
(B) Which of the elements will form covalent compounds? Give reasons. (1)
Answer:
(A) Based on the periodic table and its properties, the following questions can be answered;
(i) According to the position of Na in the periodic table, Li and K have similar chemical properties as they belong to the same group 1 and all have one valence electron.
(ii) Atomic number of N is 7 and that of P is 15. Based on this, the electronic configuration is;
N- 2,5
P- 2,8, 5
Both N and P belong to the same group as they have the same number of valence electrons. They both belong to 15th group of the periodic table.
The trend in electronegativity in the periodic table is as follows: Electronegativity increases as we move in a period from left to right and decreases as we move from top to bottom in a group.
Based on this trend, Nitrogen (N) has more electronegativity than Phosphorus (P). The reason for this is the size of the element. Nitrogen has smaller size as compared to Phosphorus. Due to this, the electrons are more closely held in the element N as compared to P.
(B) Eka-silicon is identified as germanium (Ge), which is placed in group 4 of Mendeleev’s periodic table. The valency of germanium is 4, so the chemical formula of its chloride must be GeCl4.
Eka-aluminium was later identified as gallium (Ga). It is placed in group 3 of Mendeleev’s periodic table. Hence, the valency of gallium is 3 and the formula of chloride would be GaC^.
OR
(A) Fluorine and carbon both belong to the second period. As the atomic number of C is 6, that of Fluorine is 9.
(B) Covalent compounds are formed by sharing of electrons. This property is exhibited by group 14 elements. From the given table, W will form covalent compounds as it belongs to group 14.
Section – B
Question 8.
In the given food chain, suppose the amount of energy at the fourth trophic level is 5 kJ, what will be the energy available at the producer level?
Grass → Grasshopper → Frog → Snake → Hawk (3)
Answer:
The energy available, at producers level will be 5000 Id. 90% of the energy captured (10% law) from the previous trophic level is lost to the environment, lea\/ing only 10% available to the next trophic level.
In this food chain, at the fourth trophic level, only 5kJ energy is available to the snake.
⇒ Energy available to snake = 5 kJ
⇒ Energy available to frog = 50 kJ
⇒ Energy available to grasshopper = 500 kJ
⇒ Energy available to grass = 5000 kJ.
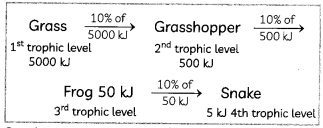
So, the energy available at the producer level will be 5000 kJ.
![]()
Question 9.
A circular metallic loop is kept above the wire PQ as shown below:

What is the direction of induced current produced in the loop, if the current flowing in the straight wire
(A) is steady, i.e. does not vary? (1½)
(B) is increasing in magnitude? (1½)
OR
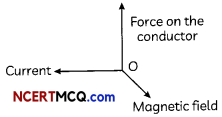
(A) State the direction of magnetic field in the following case.
(B) A boy was making a model of electric bell. He connected the coil in the circuit and switched it on. However, the magnetism produced in the coil was not strong enough. Then, he made some changes in the coil and the circuit was now working properly. He also found out other ways of producing a strong magnetism.
(i) What changes did the boy make in the coil? (½)
(ii) What values of the boy would you appreciate? (½)
(iii) Which other ways did he discover for increasing the strength of magnet? (½)
Answer:
(A) The constant current flowing in the straight wire produces a constant magnetic field. Hence, no induced current is produced in the loop.
(B) Since current in the straight wire is changing, hence, induced current will be produced in clockwise direction.
OR
(A) The direction of magnetic field is perpendicular to both current and force on the conductor, (according to Fleming’s left- hand rule).
(B) (i) The boy increased the number of turns in the coil and decreasing the gap in the air by placing the soft iron core in the coil.
(ii) Boy is a keen observer who is also experimental, curious, scientifically minded, and pragmatic.
(iii) By increasing the magnitude of current passing through the coil.
Related Theory
For induced current the change of magnetic field per unit time is must. In the absence of a changing magnetic field, induced current will not take place.
Question 10.
(A) From amongst the following sets of compounds, identify which one of them is not an alkene: C4H8, C4H6, C3H6, C5H10. (1)
(B) Identify and name the third member of the alkane family and write its formula. (1)
(C) Explain with appropriate reasons as to how many isomers are possible with the third member of the alkane family. (1)
OR
(A) Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms e.g., hydrogen. After the formation of four bonds, carbon attains the electronic configuration of which element? (1½)
(B) Why homologous series of carbon compounds are so called? Write chemical formula of two consecutive members of a homologous series and state the part of these compounds that determines their physical properties, and chemical properties. (1½)
Answer:
(A) From the given set of compounds, C4H6 do not belong to the family of alkene.
Explanation:
Members of alkene family follow the general formula CnH2n where n is an integer. On applying the formula to the given sets of compounds, we can analyse that C4H6 do not satisfy the general formula CnH2n. In C4H6, n = 4. On substituting the value of 4 in the general formula of CnH2n. we get, C4H8 instead of C4H6. Thus, C4H6 does not fall in the category of alkenes.
Related Theory:
The second homologous series is the alkenes. Their names all end in -ene, for example ethene. All alkenes contain a carbon to carbon double bond which makes them more reactive than the alkanes. The alkenes have the general formula CnH2n.
(B) The general formula of the alkane family is CnH2n+2. Here value of n = 3, thus, the formula for the third member of the alkane family is C3H8 and is called propane.
(C) Isomers are compounds with same molecular formula and different structural formula. Propane has only three carbon atoms which is not sufficient enough to exist in a branched form. Thus, propane does not exhibit isomerism.
OR
(A) The electronic configuration of carbons [2, 4]. Hence, it contains 4 valence electrons and forms 4 covalent bonds by sharing its valence electron with hydrogen. After the formation of four bonds, carbon attains the electronic configuration of neon, the nearest noble gas or inert gas with the atomic number 10. i.e., [2, 8].
(B) A homologous series is a collection of organic chemical compounds that have a similar structure and general formula (and so have comparable properties), and whose structures differ only in the number of CH2 units in the primary carbon chain. CH3Cl, and (ii) CH3CH2Cl are two consecutive members of haloalkane homologous series.
Alkyl group —CH3 and —CH3CH2 part determines physical properties while functional group —Cl determines chemical properties of the compounds.
![]()
Question 11.
(A) We are aware that each parent (mother and father) have 23 sets of chromosome each. But the child also has 23 chromosomes and not 46. Why? (1)
Answer:
A male individual has 23 pairs of chromosomes but the gamete that is formed by the meiotic cell division contain only half the number of chromosomes i.e., 23 chromosomes in male sperm and 23 chromosomes in female egg. It is the fusion of this sperm and egg which Leads to an offspring with 23 pairs of chromosomes.
(B) Why do all the gametes formed in human females have an X chromosome? (1)
Answer:
Human females have two X chromosomes. At the time of gamete formation, one X chromosome enters each gamete. Hence all the gametes possess an X chromosome.
(C) In some instances, sex is not genetically determined. Give such instances. (1)
Answer:
In some animals, the temperature at which fertilised eggs are kept determines whether the animals developing in the eggs will be male or female. In other animals, such as snails, individuals can change sex, indicating that sex is not genetically determined.
Question 12.
What is an electromagnet? Draw a circuit diagram to show how a soft iron piece can be transformed into an electromagnet. (3)
Answer:
The magnetic field produced due to current flowing in a coil or a solenoid can be used to magnetise a material Like soft iron temporarily. The insulated copper wire is wrapped on a soft iron piece. When current is passed through the coil using a battery and closing a key the iron piece behaves like a bar magnet as long as current is being passed. Such a magnet is called an electromagnet.
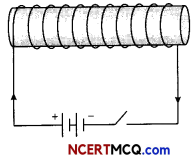
Question 13.
Classification of elements into groups and development of Periodic Law and Periodic Table are the consequences of systematizing the knowledge gained by a number of scientists through their observations and experiments.
(A) A and B are the two elements having similar properties which obey Newlands law of octaves. How many elements are there in between A and B? (1)
Answer:
Newland’s Law of Octaves states that when elements are arranged in increasing order of atomic mass, the properties of every eighth element starting from any element are a repetition of the properties of the starting element. Therefore, there will be six elements present in between A and B.
(B) An atom has electronic configuration 2, 8, 5.
(i) Calculate the number of protons. 1(1)
Answer:
The electronic configuration represents the arrangement of electrons in different shells. Thus, it also represents the total number of electrons in an element. Atomic number of an element is represented by number of electrons as well as number of protons. Thus, number of protons is equal to: 2 + 8 + 5 = 15 in the given atom.
(ii) Is it a metal or non metal? Give reason. (1)
Answer:
As we can see the electronic configuration of atom is 2, 8, 5, the element requires only 3 more electrons to attain stability. The element can gain these three electrons to attain stability. Thus, the element behaves as a non metal as it gains electrons for attaining stability.
Related Theory:
The non-metals are located on the upper right side of the periodic table. Non-metals are separated from metals by a line that cuts diagonally through the region of the periodic table. The non-metals are in a minority on the periodic table, mostly located on the right-hand side of the periodic table.
The exception is hydrogen, which behaves as a non-metal at room temperature and pressure and is found on the upper left corner of the periodic table. Under conditions of high pressure, hydrogen is predicted to behave as an alkali metal. Non-metals have high ionization energies and electronegativities. They are generally poor conductors of heat and electricity. Solid non-metals are generally brittle, with little or no metallic luster. Most non-metals have the ability to gain electrons easily. Non-metals display a wide range of chemical properties and reactivities.
Caution:
Students can get confused with the number of protons that has been asked in the question. In case ion is given instead of atom, there will be no change in the number of protons, but number of electrons will vary.
Section – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (A, B and C).
Parts A and B are compulsory. However, an internal choice has been provided in part C.
Question 14.
The reproductive parts of angiosperms are located in the flower. The different parts of a flower are sepals, petals, stamens and carpels. Stamens and carpels are the reproductive parts of a flower which contain the germ- cells. The flower may be unisexual (papaya, watermelon) when it contains either stamens or carpels or bisexual (Hibiscus, mustard) when it contains both stamens and carpels.
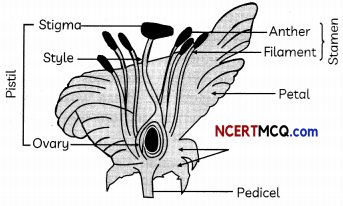
(A) Where are the plant’s sex organs located? (1)
(B) What is the function of flower? (1)
(C) (i) Where is the male and female gamete formed in flowering plants? (1)
(ii) What changes take place in the flower after fertilisation which lead to the formation of seeds and fruits? (1)
OR
Why cannot fertilisation take place in flower if pollination does not occur? (2)
Answer:
(A) Plant’s sex organs are located in the flower.
(B) The function of a flower is to make mate and female gametes and to ensure that fertilisation will take place to make new seeds for the reproduction of plant.
(C) (i) The male gemete is formed in the anther of a flower and female gamete is formed in ovry of a flower.
(ii) The fertilized egg divides several times to form an embryo within the ovule which develops a tough coat around it and is gradually converted into a seed.
The ovary the flower develops and becomes a fruit with seeds inside it.
OR
The transfer of pollen grains from the anther of a flower to the stigma of the same or another flower is known as pollination. It is done by insects birds wind and water. For the process of the fertilization, it is necessary that the male gamete reaches the female gamete. Only after the arrival of pollen grains on stigma and the entry of pollen grains on stigma and the entry of pollen tube into ovary can male gamete fuse with female gamete (ovum). This can happen when the pollen grains are transferred to the stigma through only means of pollination. If pollination does not occur, male gamete will not reach the female gamete. It can be possible through the process of pollination. Hence fertilisation cannot take place if pollination does not occur.
![]()
Question 15.
B1, B2 and B3 are three identical bulbs connected as shown in the figure. When all the three bulbs glow, a current ofSA is recorded by the ammeter A.
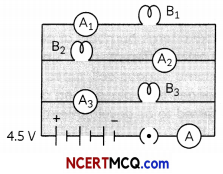
(A) What happens to the glow of the other two bulbs when the bulb Bi gets fused? (1)
(B) What happens to the reading of A1, A2, A3, and A when the bulb B2 gets fused? (1)
(C) How much power is dissipated in the circuit when all the three bulbs glow together? (2)
OR
An electric bulb is connected to a 220 V generator. The current is 0.50 A. What is the power of the bulb? (2)
Answer:
Since the bulbs are connected in parallel hence they withdraw current independently of the other bulbs.
(A) Hence when bulb Bi fuses the other two bulbs it keep on withdrawing the same current as before.
(B) When bulb B2 fuses the readings on ammeters A: and A3 remains the same as 1 Amp. But the reading of A2 becomes zero. Now since the ammeter A denotes the net current withdrawn hence the value of A becomes 2 Amp.
(C) When all three bulbs are glowing the net current withdrawn is 3A and the net voltage is 4.5V.
Hence net power dissipated is 4.5 × 3 = 13.5 W.
OR
Given:
V = 220,1 = 0.25.
Since P = VI
P = 220 × 0.25 = 55 watt
Hence power of the bulb is 55 W.