Students can access the CBSE Sample Papers for Class 10 Science with Solutions and marking scheme Term 2 Set 3 will help students in understanding the difficulty level of the exam.
CBSE Sample Papers for Class 10 Science Term 2 Set 3 with Solutions
Time allowed: 2 Hours
Maximum Marks: 40
General Instructions:
- All questions are compulsory.
- The question paper has three sections and 15 questions. ALL questions are compulsory.
- Section-A has 7 questions of 2 marks each; Section-B has 6 questions of 3 marks each, and Section-C has 2 case based questions of 4 marks each.
- Internal choices have been provided in some questions. A student has to attempt only one of the alternatives in such questions.
Question 1.
Study the part of Modern Periodic Table given below in which the alphabets represent the symbols of elements and answer the given questions:
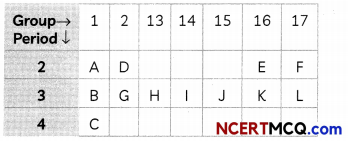
(A) Arrange the elements of 3rd period in order of decreasing metallic character. (1)
(B) Identify the elements of period 2 and group 16 which will form diatomic molecule. Draw the electron dot structure of that element. What type of bond is formed? (1)
OR
Three elements X, Y and Z with their atomic numbers are given below:
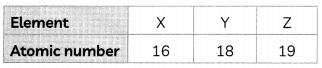
(A) Write the position of X and Z in the Modern Periodic Table. (1)
(B) Write the molecular formula formed between X and Z. (1)
Answer:
(A) The decreasing order of metallic character is B > G > H > l > J > K > L.
As we move from left to right in the periodic table, metallic character decreases and non metallic increases. Due to increase in nuclear charge the valence electrons are pulled in the strongly by the nucleus and it becomes more and more difficult for the atoms to loose electricity.
(B) Group-16 Electronic Configuration-2,6 Period-2 The element ‘E’ is Oxygen. Oxygen has 6 valence electrons, so it requires two more electron complete its octet. Hence, it will share two of its electrons with two electrons of the other oxygen atom to form a diatomic molecule O2 gas.
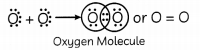
The bond formed is double covalent bond.
OR
(A) X element will be present towards the right of the table as it is a non-metal.
| Element | X |
| Electronic Configuration | 2, 8, 6 |
| Valence Electrons | 6 |
| Period | 3 |
| Group | 16(10 + 6) |
Z element will be present towards the right of the table as it is an non-metal.
| Element | X |
| Electronic Configuration | 2, 8, 8, 1 |
| Valence Electrons | 1 |
| Period | 4 |
| Group | 1 |
Explanation: The number of valence electrons determine the group number. The number of sheLLs determine the number of period. In X the number of shells are 3, so it belongs to 3rd period and has four shells so it belongs to 4th period.
(B) Valency of X is 2 and Z is 1. When X reacts with Z, Z loses one valence electron but X needs two electrons to complete its octet so there will be 2 atoms of Z which will react with X as given.
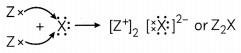
Hence, the formula of compound formed between Z and X is Z2X.
![]()
Question 2.
The following chart explains the dihybrid cross in detail between one pea plant round and green seeds and the other pea plant having wrinkled and yellow. Analyse it and answer the following questions:
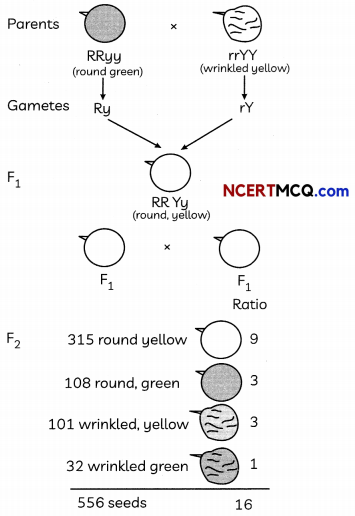
(A) In the F2 generation of a cross, progeny having different traits are produced 3:1. State whether it is a monohybrid cross or a dihybrid cross? Give one example of such a cross. (1)
Answer:
It is a monohybrid cross.
Example: when two hybrids talL Pea plants cross breed with each other they will produce three tall plants and one dwarf plant in F2 generation.
(B) Why did Mendel choose pea plant for his experiments? Give any one reason. (1)
Answer:
Mendel choose the common garden pea plant, (Pisum sativum) for his experiments because:
- It was easy to cultivate and had a relatively short life cycle of 3 months.
- The plant exhibited discontinous characteristics such as flower colour and pea texture.
- It was easy to control the self pollination of the plant and cross fertilization between desired parents could be accomplished artificially.
- Presence of pure breeding varieties and easily visible contrasting characters.
![]()
Question 3.
(A) Why do elements in a group show similar property? (1)
Answer:
All the elements present in a group have same electronic configuration of the atoms. The physical and chemical properties of elements depend on the number of valence electrons. Elements present in the same group have the same number of valence electrons. Therefore, elements present in the same group have similar physical and chemical properties.
(B) An element M is in the third group of the periodic table. Write the formulae of chloride and oxide. (1)
Answer:
Element M is in the third group, therefore it will have a valency of 3. The formula of its chloride and oxide are MCl3 and M2O3 respectively (∵ O and Cl have valencies of – 2 and – 1 respectively).
Question 4.
(A) What does the magnetic lines of force indicate? Show diagramatically. (1)
(B) What is the role of a carbon brush in a motor? (1)
OR
(A) What happens to the magnetic field strength in the presence of a ferromagnetic substance such as iron, nickle or cobalt ? (1)
(B) it is advised that a magnetic compass should not be kept for Long near a very strong magnet. Why? (1)
Answer:
(A) The magnetic field lines (lines of force) indicate the direction of magnetic field at any on the curve.
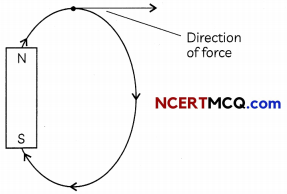
Related Theory:
Magnetic field lines show direction of magnetic field at any point on the field. Hence, if field lines cross then at point of intersection the field lines show two different directions which is clearly not possible.
(B) A carbon brush is essentially a graphite which has two important properties of electrical conduction and lubrication. The brush allows the passage of current from the external source to the rotated split rings while maintaining lubrication against wear and tear.
OR
(A) In the presence of a ferromagnetic substance the magnetic field strength increases manifold.
(B) The needle of a magnetic compass is nothing more than a magnetised needle. It’s own magnetic field strength is quite weak. On bringing it too close to a strong magnet for too long can lead to reverse magnetic induction in the compass needle which can completely change the properties of the compass.
![]()
Question 5.
Solid waste is the unwanted or useless solid material generated. Solid waste management reduces the adverse impact on the environment and human health.
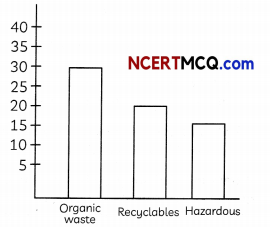
Waste composition of India in million metric tonnes per annum source AB 2016.
(A) Name a method used in hospitals for disposal of medical waste. (1)
(B) Adi practices environment friendly practices as he is an environment conscious child. Mention at least two
environment friendly practices which are practiced by you. (1)
OR
Identify the phenomenon shown in the given figure. Explain the process.
Answer:
(A) Incineration is a process used in the treatment of clinicaL wast and hazardous waste. The waste is subjected to a very high temperature to destroy the pathogens and toxic materials if present in it.
(B) It is important to follow environment friendly practices to keep the environment green and healthy. For doing so, we should stop usage of plastic bags, instead we should use bags made out of cloth and jute. We should switch off the lights and fans when they are not the use. We should restrict the use of private or personal transportation. Instead, we should either walk wherever possible or use public transport. We should not wash our cars with a hose pipe as a lot of water is wasted. Instead we should wash our cars by using bucket and mug.
OR
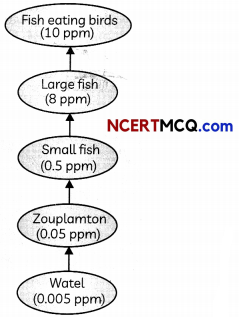
The given figure represents biological magnification in a food chain.
The accumulation of non-biodegratable pesticides such as DDT, industrial chemicals etc. in the food chain in increasing amount at each higher trophic level is biological magnification.
Related Theory:
Human beings are at the highest trophic level so they have the highest concentration of pesticides in these bodies.
![]()
Question 6.
(A) Observe the diagram of human male reproductive system and label the parts with the following functions:
(i) Production of sperms
(ii) Gland which provides fluid
(iii) Provides low temperature for the formation of sperms
(iv) Common passage for sperm and urine. (1)
Answer:
The parts of human male reproductive system performing the following functions:
- Production of sperms: Testis
- Gland which provide fluid: Prostate gland
- Provides low temperature for the formation of sperms: Scrotum.
- Common passage for sperm and urine: Urethra.
These parts or organs are labelled as follows:
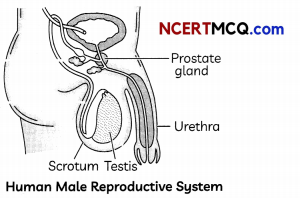
(B) Do human females also have one common passage for urine and ovum? (1)
Answer:
No, human females have two seperate passages for ovum and urine. Vaginal opening carries the ovum or fully developed foetus outside the body whereas urethra carries urine from the bladder to the outside of the body.
Question 7.
Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why? (2)
Answer:
Electronic configurations of the two elements are:
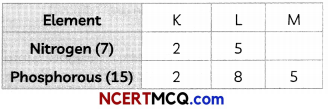
Nitrogen is more electronegative than phosphorous. On moving down in a group, the number of shells increases. Therefore, the valence electrons move away from the nucleus and the effective nuclear charge decreases. This causes the decrease in the tendency to attract electrons and hence electro-negativity decreases.
![]()
Section – B
Question 8.
Answer the following:
(A) Give an example of bisexual flower. What is its female reproductive part known as? (1)
Answer:
A flower that contains both the male and female reproductive structures (stamen and pistil) is called a biseual flower. For example: rose, Hibiscus. Its female reproductive part is known as carpel or pistil.
(B) Pollination may occur without fertlisation but fertilisation will not take place without pollination. Give reasons. (2)
Answer:
Pollination is just the transfer of pollen grains from the anther of stamen to the stigma of pistil. It is carried out by agents like wind, insect etc. For pollination to happen there is no need of fertilisation of gametes i.e. their union. Fertilisation is fusion of male and female gamete and this process is facilitated by pollination. By pollination pollens are brought to eggs so that they can fuse and fertilisation can take place. Therefore, pollination may occur without fertilisation but fertilisation will not take place without pollination.
Question 9.
(A) When green plants are eaten by herbivores, state the average amount of energy:
(i) made available for the next level of consumers.
(ii) lost to the environment (1½)
Answer:
(i) The average amount of energy mode available for the next level of consumers is 10% of the. available energy present in green plants.
(ii) The average amount of energy lost as heat to the environment is 90%.
(B) Nowadays, our government is stressing upon the use of jute or paper bags instead of plastic bags. What purpose is supposed to be achieved by the government? (1½)
Answer:
Jute bags or paper bags are prepared from biodegradable materials thus lowering the environmental pollution. Plastic bags are non-biodegradable and affect the environment adversely.
![]()
Question 10.
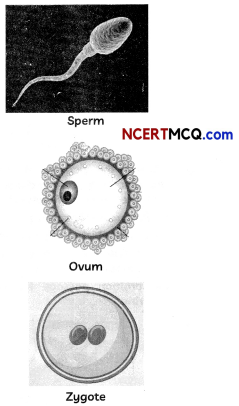
(A) Observe the given figures and distinguish between o gamete and zygote. (1½)
(B) Explain their rotes in sexual. reproduction. (1½)
OR
(A) Identify the type of asexual reproduction in the following organisms. (1½)

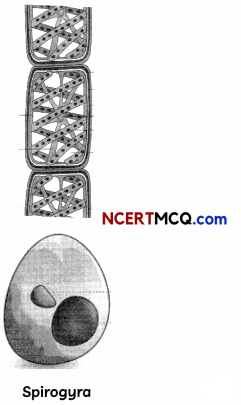
(B) List four advantages of vegetative propagation. (1½)
Answer:
(A) Differences between a gamete and zygote:
| Gamete | Zygote |
| Gametes are germ cells that are mixed during sexual reproduction, such as sperm (male) and ova (female). | Zygote is the result of the sexual reproduction of male and female gametes fusing together. |
| Unfertilized reproductive cells are known as gametes. These are haploid cells. | Zygote is a fertilized egg or a fertilised egg and is diploid. |
| Gametes contain 22 autosomes, and 1 sex chromosome, either X or Y. | Zygote contain 22 pairs of autosomes, and 1 pair of sex chromosome, either XX or XY. |
(B) Role of gamete and zygote in sexual reproduction: Gametes are the result of meiosis, they have half the number of chromosomes as the parent cells. During fertilisation and zygote formation, the number of chromosomes is restored.
As a result, the gamete and zygote are two phases of sexually reproducing organisms that help to keep the number of chromosomes in each species constant.
OR
The type of asexual reproduction in the given organism are:
(A) (a) Sugarcane – vegetative propagation by stem cutting
(b) Spirogyra – Fragmentation
(c) Yeast – Budding
(B) Advantages of vegetative propagation:
- The new plants produced will be exactly same like the parent plants.
- The trees grown from cutting or grafting start to bear fruits much earlier as compared to the trees that grow from seeds.
- Many plants can be grown from just one parent plant by this method.
- We can also get seedless plants by this method.
![]()
Question 11.
What is JouLe’s heating effect? How can it be demonstrated experimentally? List its applications in daily life. (3)
Answer:
Joule’s heating effect: When an electric current is pased through a high resistance metallic wire, like nichrome wire the resistance wire becomes very hot and produces heat. This effect is known as heating effect of current or Joule’s heating effect.
Joule’s law of heating states that the heat H produced in a conductor of resistance R due to current flowing through it for time t is H = I2Rt.
A simple experiment to demonstrate heating effect of current is that if we switch on the bulb for a long period of time then it will become hot. This shows that when electric current flows through a metallic conductor, heat is produced in it.
Applications of Joule’s heating effect in daily life are:
- Electric fuse is a safety circuit device which works on this principle. ELectric fuse in the electric circuit melts when large current flows in the circuit.
- Electric iron, electric heater and water heater, etc. works on the principle of heating effect of current.
- Electric bulb glows when electric current flows through the filament of the bulb.
Question 12.
Four resistors of 2Ω each are joined end to end to form a square PQRS. Calculate equivalent resistance of the combination between any two adjacent corners. (3)
OR
(A) A current of 1 A is drawn by a filament of an electric bulb. Calculate the number of electrons passing through a cross section of the filament in 16 seconds. (1½)
(B) Should the resistance of an ammeter be low or high? Give reason. (1½)
Answer:
The circuit diagram will be:
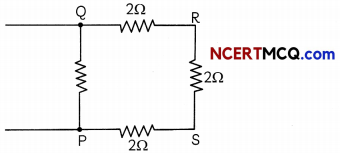
Let us consider the equivalent resistance between points P and Q.
Here, three resistances R2(QR), R3(RS), R4(SP) of 2 ohm each are in series, and this combination is connected in parallel across the resistor R1 of 2 ohm (PQ) (R1 = 2 ohm)
Equivalent resistance of the three series resistors.
R = R2 + R3 + R4
= 2 + 2 + 2 = 6 ohms
Thus, required equivalent resistance (R)
\(\frac{1}{R}\) = \(\frac{1}{R_{1}}+\frac{1}{R}\) = \(\frac{1}{2}+\frac{1}{6}\) = \(\frac{4}{6}\)
R = 1.5 ohm.
OR
(A) Electric current l = 1A
Time t = 16 seconds
No of electrons n = ?
We know that current is the amount of electric charge passing through a given point of conductor in 1 second.
l = \(\frac{Q}{t}\) = \(\frac{n e}{t}\)
⇒ n = \(\frac{\text { It }}{e}\) = \(\frac{1 \times 16}{1.6 \times 10^{-19}}\)
⇒ n = 10 × 1019
= 1020 electrons
The number of electrons flowing is 1020 electrons.
(B) An ideal ammeter is one which has zero resistance. But that is not possible. Therefore, the resistance of an ammeter should be as cLose to zero as possible. It it is non zero and substantial, it will affect the current flowing through the circuit. This is because an ammeter is connected in series in the cirucitforthe measurement of electric current.
![]()
Question 13.
A persan P has only Q chromosomes in all its gametes. On the other hand, another person has chromosome S in half of gametes and chromosome T in another half of gametes When chromosomes Q and S combine during fertilization, a female zygote results. On other hand, combination of Q and T chromosomes produces a male zygote.
(A) What are chromosomes
(i) Q
(ii) S
(iii) T?
Answer:
As a woman has only X chromosomes in all her gametes and it is given that person P has on Q chromosomes in all its gametes, thus, Q is ‘X’ chromosome.
It is also given that another person R has chromosome S in half of gametes and chromosoms T in another half of gametes. As we know that males have X chromosomes in half gametes and Y chromosomes in another half, thus, person R is a male.
When chromosome Q and S combine during fertilisation, a female zygote results. It means that both Q and S are X chromosomes.
When Q and T chromosomes combine, a male zygote results which means that while Q is chromosome. T is Y chromosome. Therefore Q is X chromosome, S is X chromosome and T is Y chromosome.
(B) Out of Q, S and T, which 2 chromosomes are of the same type? (½)
Answer:
Out of Q, S and T, Q and S chromosomes are of the same type, namely X chromosome.
(C) Out of the two persons P and R, which one is male and female? Give reasons for alt your answers. (½)
Answer:
Y chromosomes are similar in size so T is smaller in size.
Q and T are XY sex chromosomes present in males.
Out of P and R, P is a female as it has only X chromomes in all gametes and R is a male as it has X chromosomes in half of the gametes and Y in the other half.
Section – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (A, B and C).
Parts A and B are compulsory. However, an internal choice has been provided in part C.
Question 14.
Hydrocarbons are organic compounds that contain only carbon and hydrogen. The broadest distinction between hydrocarbons is whether they are saturated and unsaturated.
The four distinct hydrocarbon functional groups are: alkanes, alkenes, alkynes and arenes.
(A) You are given a bails and stick model of six carbon atoms and fourteen hydrogen atoms and sufficient number of sticks. In how many ways one can join the models of six carbon atoms and fourteen hydrogen atoms to form different molecules of C6H14. (1)
(B) Give the electron dot structure of C3H6O which is an aldehyde. (1)
(C) (i) In an organic compound, which parts largely determine its physical and chemical properties? (1)
(ii) The molecular formula of two carbon compounds are C4H8 and C3H8. Which one of the two is more reactive? Justify. 1
OR
(i) Identify the number of single covalent bonds in ethanol, C2H5OH. (1)
(ii) What category of compounds are formed when hydrogen is replaced by hydroxyl? (1)
Answer:
(A) There are 5 possible ways in which hexane can be arranged as shown below:
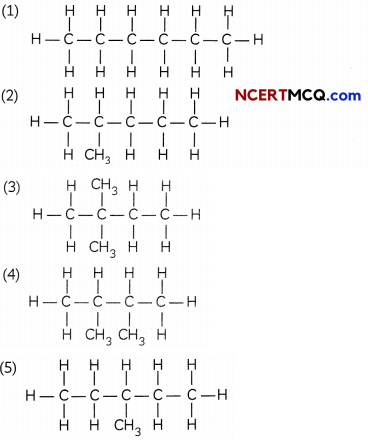
(B)
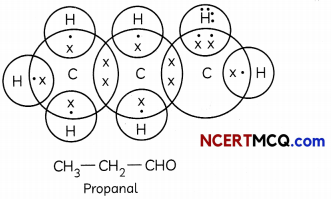
(C) (i) Functional group determines chemical properties while alkyl chain determines physical properties.
Related Theory:
Functional groups refer to specific atoms bonded in a certain arrangement that give a compound certain physical and chemical properties. Functional groups can used to distinguish similar compounds from each other.
(ii) Among C4H8 and C3H8, the compound having molecular formula C4H8 is an unsaturated hydrocarbon having general formula CnH2n, while C3H8 is a saturated hydrocarbon having the general formula CnH2n+2. Unsaturated hydrocarbons are more reactive as compared to saturated hydrocarbon, thus, C4H8 will be more reactive than C3H8.
Related Theory:
The unsaturated hydrocarbons are more reactive due to the presence of double and triple bonded carbon atoms as these are weaker than the single bonded saturated hydrocarbons due to the presence of weaker pi bonds and thus, when a reaction takes place, these unsaturated hydrocarbons break down easily as compared to saturated hydrocarbon.
OR
(i) The skeletal structure for ethanol is
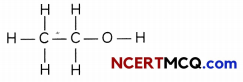
Based on this, the number of single covalent bond is:
C-H bonds: 5
C-C bonds : 1
C-0 bonds: 1
O-H bonds: 1
Total number of single covalent bonds: 8
(ii) When hydrogen is replaced by hydroxyl group, the resultant compound formed is an alcohol.
![]()
Question 15.
Let’s say you have a metal rod, and decide to connect that to your galvanometer. If the rod is stationary in a magnetic field, nothing happens. If you move that rod through the field however, an emfis induced between the ends of the rod causing current to flow. This is because when you move the metal rod through the field, you are moving all the rod in the rod. These moving charges are deflected by the field toward one end of rod creating a potential difference. This is known as motional emf.
(A) State the rule that determines the direction of induced emf state. (1)
(B) What is the relation between the direction of induced emf and the direction of motion of conductor? (1)
(C) (i) Examine the following graphs show
the effect of changing the speed of rotation of the coil on the induced emf: and write your interpretations. (1)
(ii) What is electromagnetic induction? (1)
OR
(i) Identify the V-l graphs for ohmic and non-ohmic materials. (1)
(ii) Give one example of each. (1)
Answer:
(A) The direction of induced current is given by Fleming’s Right hand rule whereas Fleming’s left hand rule is used to find the direction of force on a current carrying conductor placed in a magnetic field. Faraday’s law is used to explain the phenomenon of electromagnetic induction. Right hand thumb rule is used to find the direction of magnetic field around a current carrying conductor.
(B) According to Fleming’s right hand rule the induced emf the motion of the conductor and the magnetic flux are mutually perpendicular.
(C) (i) Both the magnitude and frequency of induced emf will be increased on increasing the coil rotation speed.
If the speed of rotation of the coil is changed two things happen:
- Since the rate of threading the magnetic field lines is increased the output emf wiLl also be increased.
- The frequency of the output emf will be increased as well since the coil makes a revolution in a shorter time.
(ii) The phenomenon of inducing current in a coil by a changing magnetic field is called electromagnetic induction. Current is induced in a conductor or coiL when there is a relative motion between the coil and the magnet.
OR
(i)
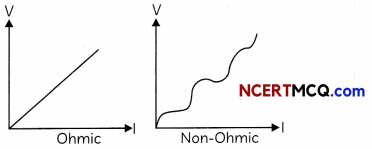
(ii) Ohmic: Conductors,
Non: Ohmic – Semi-Conductors