CBSE Sample Papers for Class 9 Science Paper 7 are part of CBSE Sample Papers for Class 9 Science. Here we have given CBSE Sample Papers for Class 9 Science Paper 7.
CBSE Sample Papers for Class 9 Science Paper 7
| Board | CBSE |
| Class | IX |
| Subject | Science |
| Sample Paper Set | Paper 7 |
| Category | CBSE Sample Papers |
Students who are going to appear for CBSE Class 9 Examinations are advised to practice the CBSE sample papers given here which is designed as per the latest Syllabus and marking scheme as prescribed by the CBSE is given here. Paper 7 of Solved CBSE Sample Paper for Class 9 Science is given below with free PDF download solutions.
Time Allowed : 3 Hours
Max. Marks: 80
General Instructions
- The question paper comprises of two Sections, A and B. You are to attempt both the sections.
- All questions are compulsory. However an internal choice will be provided in two questions of 3 marks each and one question of five marks.
- All questions of Section A and all questions of Section B are to be attempted separately.
- Question numbers 1 to 2 in Section A are one-mark questions. These are to be answered in one word or in one sentence.
- Question numbers 3 to 5 in Section A are two-marks questions. These are to be answered in about 30 words each.
- Question numbers 6 to 15 in Section A are three-marks questions. These are to be answered in about 50 words each.
- Question numbers 16 to 21 in Section A are five-marks questions. These are to be answered in about 70 words each.
- Question numbers 22 to 27 in Section B are two-marks questions based on practical skills. These are to be answered in brief
Questions
SECTION-A
Question 1.
Define saturated solution.
Question 2.
What is plasma membrane made up of?
Question 3.
What would happen to the cell if there is no Golgi apparatus?
Question 4.
During free fall what is the weight of the body? Give reasons for the answer.
Question 5.
The relative density of gold is 19.3. The density of water is 103 kg/m3? What is the density of gold in S.I. unit?
Question 6.
Two block pieces of same mass and same size are dipped in water and oil. One block floats in water and the other sinks in oil. Explain why? When you mix oil and water, explain what .will be the top layer of the mixture.
Question 7.
Write the electronic configuration and valency of the following:
- Fluorine
- Potassium
- Silicon
OR
You are given an element \(_{ 17 }^{ 35 }{ X }\) .
- Find the number of protons, electrons and neutrons.
- Valency of X.
- Write the chemical formula of the compound formed when it combines with carbon and sodium.
Question 8.
Why is ice at 273 K more effective in cooling than water at the same temperature?
OR
How would you confirm that a colourless liquid given to you is pure water? Give three tests.
Question 9.
Give the function of the following: nuclear membrane, mitochondria, ribosomes.
Question 10.
Classify the following animals into their phyla giving, one reason:
- Earthworm
- Scorpion
- Starfish
Question 11.
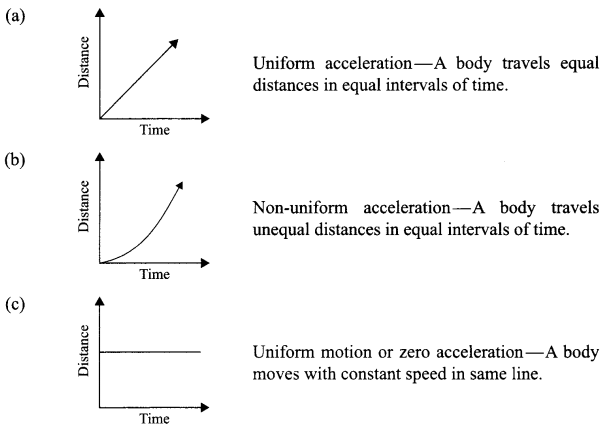
Deine by drawing the graphs to show the following:
(a) Uniform acceleration
(b) Non-uniform acceleration
(c) Uniform motion
Question 12.
- Define atomic mass unit.
- Distinguish between molecular mass and molar mass.
- Deine and give one example of
- diatomic and
- triatomic molecule of compounds.
Question 13.
- Explain why it is dangerous to jump out of a moving bus.
- Explain why a sportsman gives a swing to his arm while catching a cricket ball.
- How does a karate player break a slab of ice with a single blow.
Question 14.
A radio station is transmitting its signals at a frequency cf 400 MHz. If the velocity of radio wave is 3 × 108 m/s, calculate the wavelength of radio waves.
Question 15.
Define power. Write is its S.I. unit. An electric bulb is rated 15 watts. What does it mean? What is the energy consumed in joules if it is used for 10 minutes?
Question 16.
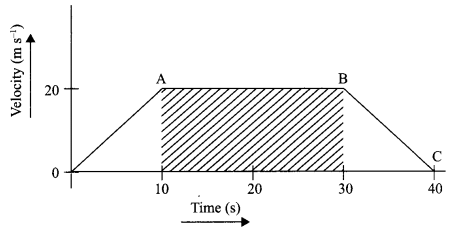
The velocity time graph of a body is given as follows:
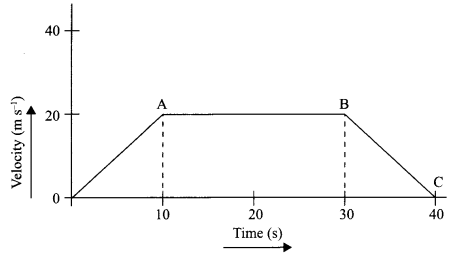
(i) State the kind of motion represented by OA; AB.
(ii) What is the velocity of the body after 10 s and after 40 s?
(iii) Calculate the retardation of the body.
(iv) Calculate the distance covered by the body between 10th and 30th second.
Question 17.
- Define vaccine. Name two vaccines given to the children.
- What is antibiotic penicillin? Give its function.
Question 18.
How can crop variety improvement methods come to the rescue of farmers facing repeated crop failures? Describe three factors for which they could do crop improvement.
Which is the most common method of obtaining improved variety of crops? Explain briefly.
Question 19.
Explain ‘potential energy’ in your own words and give an example of it. State the S.I. unit of potential energy. Write the expression for the potential energy of an object of mass ‘m’ that has been raised to a height ‘h’ from the ground.
A body of mass 20 kg is lifted up by 10 meters. Calculate its potential energy. If this body is allowed to fall freely, find its kinetic energy just before it touches the ground. (Take g= 10 m/s2)
Question 20.
(a) Distinguish between true solution, suspension and colloids in a tabular form under the following headings:
(i) Stability
(ii) Filterability
(iii) Type of mixture
(b) What is meant by concentration of a solution? How will you prepare a 10% solution of glucose in water?
Question 21.
- State one similarity and one difference between evaporation and boiling.
- List four factors which affect the rate of evaporation.
- Describe an activity to show that water vapour is present in air.
SECTION-B
Question 22.
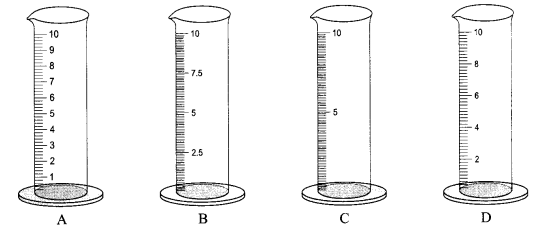
Four measuring cylinders with different least counts are shown in figures A, B, C and D. Name the most suitable cylinder for determining the volume of a cube of side 1 cm and why?
Question 23.
A teacher soaked 10 raisins in 35 mL of distilled water in a beaker labelled A and perform a similar activity and label the other beaker B. She maintained the temperature of beaker A at 20°C and B at 40°C. After an hour which one has higher percentage of water absorbed by raisins, beaker A or by those in beaker B?
Question 24.
Three students A, B and C determined the volume of a solid by immersing it in water in the overflow cans set-up as shown.
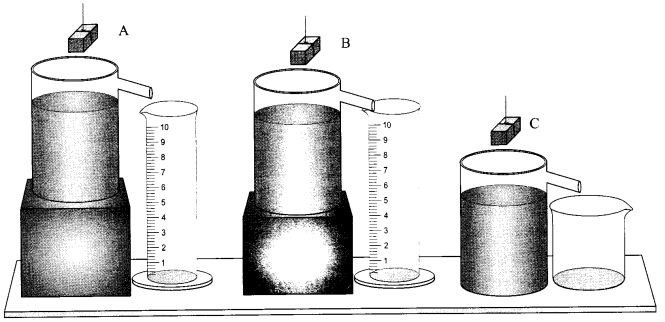
In which of these cases the result obtained will be correct/incorrect and why?
Question 25.
How does a cockroach adapt itself to wide range of habitats?
Question 26.
What are the different adulterants commonly used in foods?
Question 27.
Two bottles of equal volume are filled with glycerin and water respectively. Which of the bottle will be heavier? Give reason for your answer.
Answers
SECTION-A
Answer 1.
The solution in which no more solute can be dissolved at given temperature.
Answer 2.
Prateins and Lipids
Answer 3.
Golgi apparatus has the function of storage, modification and packaging of the products in vesicles. If there were no Golgi bodies, packaging and dispatching of materials synthesised by the cell will be stocked and it remained unclear where the proteins or synthesised materials will go.
Answer 4.
No weight, as the body is experiencing the force due to gravity.
Answer 5.
Relative density of gold = 19.3
Relative density of gold = \(\frac { Densityofgold }{ Densityofwater }\)
∴ Density of gold = Relative density of gold × Density of water
= 19.3 × 103 kg/m3
= 19300 kg/m3
Answer 6.
The density of water is more than oil. Hence the buoyant force exerted by water on the object is more than by oil. Therefore among two identical pieces, one piece will float in water and the other sinks in oil.
Oil will float on water as its density is less than water. Therefore, oil will form the topmost layer.
Answer 7.
- Flourine: Atomic number = 9; Electronic configuration = 2,1 and Valency = 1
- Potassium: Atomic number = 19; Electronic configuration = 2, 8, 8, 1 and Valency = -1
- Silicon: Atomic number = 14; Electronic configuration = 2, 8, 4 and Valency = 4
OR
- Protons = 17, Electrons = 17 and Neutrons = 35 – 17 = 18
- Valency of X is 1.
- CX4 and NaX
Answer 8.
At 273 K or 0°C, ice will have more cooling than water because it can absorb more heat than water due to its latent heat of fusion. Water at the same temperature does not hold the latent heat of fusion, that is the hidden heat which is present in ice and thus it cannot absorb heat more than ice and will have less cooling effect.
OR
- Boiling test of water: Pure water has fixed boiling point of 100 degree Celsius. If a liquid boils at this temperature, then it is water.
- On boiling a sample of water all the water should evaporate and nothing is left behind, no salt or residue.
- pH test and freezing point: The pH of water is 7, freezing point of water is 0 degree Celsius. If a liquid freezes at 0 degrees, then it is water.
Answer 9.
- Nuclear membrane: It is a membrane made up of double layer of lipid and it is also called as the envelope that protects the nucleus in the cell.
- Mitochondria: It is called the powerhouse of the cell as it stores energy in the form of ATP. It regulates the metabolism.
- Ribosomes: It helps in making of proteins in the cell. Protein is needed for many cell functions such as repairing, damage or directing the chemical processes.
Answer 10.
- Earthworm: Annelids, as their body is segmented.
- Scorpion: Arthropoda, as it has joint appendages.
- Starfish: Echinodermata, as it has calcareous skeleton and tube feet.
Answer 11.
Answer 12.
- Atomic mass unit: A unit of mass used to express atomic and molecular weights, equal to one twelfth of the mass of an atom of carbon-12 isotope.
- Molecular mass: Molecular mass of a compound is the mass of of its one molecule. This is measured in atomic mass unit (amu). 1 atomic mass unit is the one twelfth of the mass of an atom of C-12 isotope. When the mass of a molecule is divided by the mass of one twelfth of the mass of an atom of C-12 isotope, its relative mass is obtained.
- Molar mass: This is also known as molecular weight. It is the mass of a substance for a given amount. The S.I. unit for the molar mass is g mol-1. This gives the amount of the atoms/molecules/ions present in one mole of a substance.
-
- Diatomic molecule of compound: A molecule of a compund with two atoms, e.g. MgO, NaCl.
- Triatomic molecule of compound: A molecule of a compound with three atoms, e.g. CO2, H2O.
Answer 13.
- While moving in a bus our body is in the state of motion. On jumping out of a moving bus our feet touches the ground and came to rest, while the upper part of our body stays in motion and moves forward due to inertia of motion and hence we can fall in forward direction.
- While catching a fast moving cricket ball, a fielder on the ground gradually pulls his hands backwards with the moving ball. This is done so as to increase the time during which the high velocity of the moving ball decreases to zero. Thus, the acceleration of the ball is decreased and so the impact of catching the fast moving ball is reduced.
- A karate player applies the blow with a large velocity in a very short interval of time on the ice slab, which therefore exerts a large amount of force on it and suddenly breaks the ice slab.
Answer 14.
Frequency of the signals = 400 MHz
= 400 × 106 Hz
Velocity of the waves = 3 × 108 m/s
Wavelength of the radio waves = ?
We know, Speed = Wavelength × Frequency
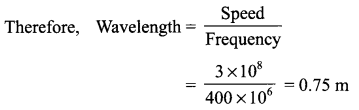
Answer 15.
Power is the rate of doing work i.e. the amount of work done per unit time.
S.I. unit: Watt
If a bulb is rated 15 watts, it means it consumes 15 joules per second.
Therefore, power = \(\frac { Energy }{ Time }\)
So, Energy = Power × Time
Therefore energy consumed by the bulb is 15 × (10 × 60) seconds
= 15 × 600
= 9000 Joules
Answer 16.
(i) OA – uniform acceleration, AB – zero acceleration/constant velocity.
(ii) 20 ms-1 : zero/body comes to rest.
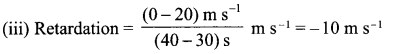
(iv) Distance between 10th and 30th second = area of figure shaded
= (30 – 10)s × 20 ms-1
= 20 s × 20 m s
= 400 m
Answer 17.
- Vaccine is a chemical/drug given in advance to an individual to give immunity to the body against certain diseases.
Two vaccines given to children are:- BCG – for tuberculosis prevention
- Polio drops – for polio prevention
- Penicillin is an antibiotic that blocks the bacterial processes to build the cell wall. Due to this drug, the bacteria is unable to make a protective cell wall and dies easily. It is used to cure the diseases and infections caused by bacteria.
Answer 18.
Crop variety improvement methods came to the rescue of the farmers, facing repeated crop failures in the following ways:
- Improved quality of crops are obtained in higher yield.
- The cost of production is reduced & there is uniform maturity of crops which makes harvesting process easier & reduces loss during harvesting.
- Crops with desirable agronomic characteristics are obtained having wider adability to the climatic conditions.
- Soil fertility is maintained and there is no or minimum weeds.
Three factors for which farmers can do crop variety improvement are:
- Develop biotic and abiotic resistance
- Wider adaptability
- Early and uniform maturity
Most common method is Hybridisation, which involves crossing between two varieties having genes for the desired characteristics and bringing them together into a new variety called the hybrid.
Answer 19.
‘Energy possessed by an object by virtue of its position or configuration. ’ (To be explained in candidate’s own words.)
Example: Stretched string of bow/ a stone lifted to a certain height (or any other appropriate example).
S.I. unit – Joule
Potential Energy, PE. = mgh
As the object falls, m = 20 kg g = 10 m/s2 h = 10 m
∴ P.E = mgh
= 20 kg × 10 m/s2 × 10 m
P.E = 2000 J
Its P. E. gets converted to K. E.
Kinetic energy just before it touches = 2000 J
Answer 20.
(a)
| Property | Solution | Suspension | Colloid |
| Stability | Stable, i.e., particles do not settle down on keeping. | Not stable, i.e., particles settle down on keeping. | Stable, i.e., particles do not settle down on keeping. |
| Filterability | Passes through the filter paper-particle size is very small. | Suspended particles do not pass through the filter paper- particle size is large. | Passes through the filter paper-particle size is small. |
| Type of mixture | Homogeneous | Heterogeneous | Heterogeneous but appears to be homogeneous |
(b) Concentration of a solution is the amount of the solute present in a given amount (mass or volume) of solution (or solvent).
Dissolve 10 g or glucose in 100 g – 10 g = 90 g of water
Answer 21.
1. Similarity: Liquid state is changed into of a substance its gaseous state.
| Evaporation | Boiling |
| It is a surface phenomenon, i.e., water molecules at the surface gain energy to change their state. | It is a bulk phenomenon. All (bulk) the water molecules at a time gain energy to change their state. |
2. Four factors:
- Surface area of the liquid exposed to the atmosphere
- Temperature of the liquid
- Humidity
- Wind velocity
- Vapour pressure of the liquid (any four)
3. When ice cold water or crushed ice is taken in a tumbler, water droplets soon appears on the outer surface of the tumbler./Anhydrous calcium chloride turns wet on keeping exposed to air. (Any one)
SECTION-B
Answer 22.
Cylinder A with the minimum least count will be used to find the volume of a cube with very small dimensions.
Answer 23.
The raisins soaked in B will have absorbed more water as the temperature of water is higher and as the osmosis process will be more in B than in A, since the temperature of water in A is less.
Answer 24.
In all the three cases the result obtained will be incorrect. This is because the water in the overflow can should be filled upto the outlet brim.
Answer 25.
Cockroach has a hard exoskeleton that serves many functions, including protection from water loss and physical injury and internally aids in muscle attachment, which results in tremendous leverage and strength. It also undergoes hibernation during unfavourable conditions.
Answer 26.
The commonly used adulterants in food are:
- Black pepper: dry seeds of papaya
- Honey: jaggery
- Red chilli powder: red brick powder
- Mustard seed: argemone seeds
Answer 27.
The bottle with glycerine would be heavier because its mass is greater than water. The density of a substance is directly proportional to the mass of the body. The density of glycerine is 1.26 g/cc and that of water is 1 g/cc.
We hope the CBSE Sample Papers for Class 9 Science Paper 7 help you. If you have any query regarding CBSE Sample Papers for Class 9 Science Paper 7, drop a comment below and we will get back to you at the earliest.