Chemical Reactions:
We come across a variety of changes that take place around us in our daily life. These changes may be physical changes or chemical changes.
In this chapter we will study chemical reactions and their characteristics, balancing chemical equations, various types of chemical reactions such as combination reactions, decomposition reactions, displacement reactions, double decomposition reactions, exothermic and endothermic reactions, redox reactions and effects of oxidation reactions in our daily life.
Physical changes are the changes which bring about changes in physical characteristics of a substance without changing the chemical composition of the substance.
Examples: Melting of ice to form water, boiling of water to form water vapour, dissolving salt or sugar in water, mixing carbon dioxide gas in soda etc.
Chemical changes are the changes in which new substances are formed having properties entirely different from the original substance(s).
![]()
Examples: Cooking of food, digestion, respiration, burning of coal, rusting of iron, souring of milk etc. Chemical reactions are the processes involving a chemical change in which new substances having new properties are formed from original substances.
Chemical reactions involve breaking of old bonds and formation of new bonds.
Reactants and Products:
The substances which take part in a chemical reaction are called reactants while the new substances produced as a result of chemical reaction are called
products.
Examples:
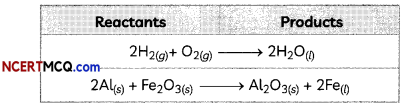
Characteristics of Chemical Reactions
A chemical reaction is characterized by any of the following observations:
Change in State:
Consider the reaction between ammonia gas with
hydrogen chloride gas to produce solid ammonium chloride. Change of state takes place in this reaction.
NH3(g) + HCl(g) → NH4Cl(s)
Change in Colour:
In the reaction between silver (silvery white in colour) and hydrogen sulphide to form silver sulphide (black) and hydrogen gas, change of colour takes place.
Evolution of a Gas:
The reaction between zinc and dilsulphuric acid is characterized by the evolution of hydrogen gas.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Change in Temperature:
A chemical reaction can be exothermic or endothermic in nature.
The reaction between zinc and dil. sulphuric acid to form zinc sulphate and hydrogen gas is an exothermic reaction as heat is evolved.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) + Heat
However, the reaction between ammonium chloride and Barium hydroxide is an endothermic reaction as heat is absorbed.
Ba(OH)2(aq) + 2NH4Cl(s) + Heat → BaCl2(aq) + 2NH3 + 2H2O(l)
Formation of a Precipitate
When barium chloride solution reacts with sulphuric acid, barium sulphate is formed which is an insoluble white substance also known as precipitate, along with hydrochloric acid.
BaCl2(oq) + H2SO4(oq) → BaSO4(s) ↓(White ppt) + 2HCl(aq)
Example 1.
Case Based:
A 2 cm long thin ribbon of a metal X was taken and first cleaned with sandpaper. It was then burnt using a spirit lamp or burner by holding it with a pair of tongs. The ribbon burnt with a dazzling white flame and formed a powder Y which was collected in a watch glass.
(A) Which option correctly identifies both X and Y?
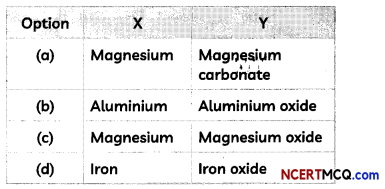
Answer:
(c) X is magnesium and Y is magnesium oxide.
Explanation: When magnesium ribbon is burnt, it forms a white powder of magnesium oxide (MgO) due to the reaction between magnesium and oxygen present in the air.
2Mg + O2 → 2MgO
(B) The colour of powder or ash formed when a magnesium ribbon is burnt in the air is:
(a) grey
(b) black
(c) white
(d) yellow.
Answer:
(c) white
Explanation: When a magnesium ribbon is burnt in air, it forms magnesium oxide, which is a white coloured powder.
(C) Why should a magnesium ribbon be cleaned before burning in air?
Answer:
Magnesium ribbon should be cleaned before burning in air to remove the layer of magnesium oxide that may have formed on the ribbon due to the reaction of magnesium with oxygen.
![]()
(D) Is burning of magnesium ribbon a physical change or a chemical change? Justify your answer.
Answer:
Burning of ribbon is a chemical change as magnesium burns in air to form a new substance magnesium oxide. Moreover, it also involves change in temperature as a lot of heat and light is produced during this change.
(E) Assertion: Magnesium ribbon burns with a dazzling white flame.
Reason: When magnesium ribbon burns in air only heat is evolved.
(a) Both (A) and (R) are true, and (R) is correct explanation of the assertion.
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the assertion.
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Answer:
(c) (A) is true, but (R) is false.
Explanation: Magnesium ribbon burns in presence of air to form magnesium oxide. A Large amount of heat and light are produced due to which we see a dazzling white flame.