Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 13 Amines. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Amines MCQs Pdf with Answers to know their preparation level.
Amines Class 12 Chemistry MCQs Pdf
1.

Answer/Explanation
Answer: d
Explaination:
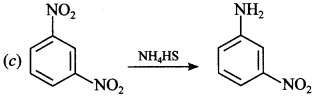
2. The major product of the reaction between /n-dinitro benzene with NH4HS is
(a) p-Dinitro benzene
(b) m-Diamino benzene
(c) m-nitroaniline
(d) p-Diamino benzene
Answer/Explanation
Answer: c
Explaination:
3.

Answer/Explanation
Answer: d
Explaination:

4. An organic compound C7H9N forms clear solution when dissolved in KOH after reacting with C6H5SO2Cl, ‘A’ on diazotisation at 0°C and then reaction with β-naphthol gives orangish red dye. ‘A’ on electrophilic substitution gives single product. ‘A’ is
(a) 4-Methyl aniline
(b) 2-Methyl aniline
(c) 3-Methyl aniline
(d) N-Methyl aniline
Answer/Explanation
Answer: a
Explaination:

5.

(a) β-Alanine
(b) α-Alanine
(c) Ethylene diamine
(d) Oxamide
Answer/Explanation
Answer: a
Explaination:

6. The reaction of p-Toluidine with CHCl3 and KOH gives.

Answer/Explanation
Answer: d
Explaination:
(d) It is carbylamine reaction, leads to formation of isocyanide (carbylamine).
7.
![]()
The compound ‘A’ is
(a) propane nitrile
(b) ethane nitrile
(c) nitro methane
(d) methyl isocyanate
Answer/Explanation
Answer: b
Explaination:
![]()
8.
![]()
The IUPAC name of ‘Y’ is
(a) N-isopropyl methanamine
(b) N-methyl propan-2-amine
(c) N-methyl propanamine
(d) butan-2-amine
Answer/Explanation
Answer: b
Explaination:

9.

Answer/Explanation
Answer: a
Explaination:
(a) sp > sp² > sp³. In CH3C≡N, ‘N’ is sp hybridised, in pyridine sp² hybridised and (CH3)3N, it is sp³ hybridised.
10. The best reagent for converting 2-Phenyl propanamide into 2-phenyl propanamine is
(a) Br2/NaOH
(b) excess of H2
(c) I2/P4
(d) LiAlH4 in ether
Answer/Explanation
Answer: d
Explaination:

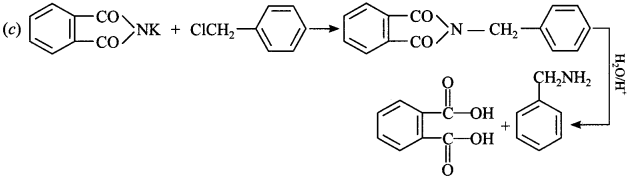
11. Which one of the following can be prepared by Gabriel phthalimide synthesis?
(a) Aniline
(b) o-Toluidine
(c) Benzylamine
(d) N-Methyl ethanamine
Answer/Explanation
Answer: c
Explaination:
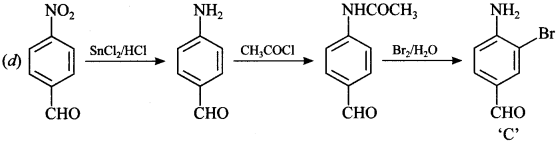
12.

Answer/Explanation
Answer: a
Explaination:

13. Diethyl amine, when treated with HN03 gives
(a) Diethyl ammonium nitrite
(b) Ethly alcohol
(c) N-nitroso diethyl amine
(d) Triethyl ammonium nitrite
Answer/Explanation
Answer: c
Explaination:
![]()
14. Choose the amide which on reduction with LiAlH4 gives secondary amine.
(a) Ethanamide
(b) N-Methyl ethanamide
(c) N, N-diethyl ethanamide
(d) Benzamid
Answer/Explanation
Answer: b
Explaination:

15. 4-Nitrotoluene is treated with bromine to get P. ‘P’ is reduced with Sn/HCl to get compound ‘Q\ ‘Q’ is diazotised and the product is treated with phosphinic acid to get compound ‘R’. ‘R’ is oxidised with alkaline KMn04 to get ‘S’. Compound ‘S’ is
(a) 2-Bromo-4-hydroxy benzoic acid
(b) 2-Bromo benzoic acid
(c) 3-Bromo benzoic acid
(d) 4-Bromo benzoic acid
Answer/Explanation
Answer: b
Explaination:

16. In order to prepare a 1° amine from an alkyl halide with simultaneous addition of one CH2 group in the carbon chain, the reagent used as source of nitrogen is ____________ . [NCERT Exemplar]
(a) Sodium amide, NaNH2
(b) Sodium azide, NaN3
(c) Potassium cyanide, KCN
(d) Potassium phthalimide, C6H4(CO)2N–K+
Answer/Explanation
Answer: c
Explaination:
(c) KCN is used to increase number of carbon atoms.
17. Amongst the given set of reactants, the most appropriate for preparing 2° amine is ____________ . [NCERT Exemplar]
(a) 2° R—Br + NH3
(b) 2° R—Br + NaCN followed by H2/Pt
(c) 1° R—NH2 + RCHO followed by H2/Pt
(d) 1° R—Br (2 mol) + potassium phthalimide followed by H3O+/heat
Answer/Explanation
Answer:
Explaination:

18. The order of basic strength of amines in aqueous solution is
(a) (CH3)3N > (CH3)2NH > CH3NH2 > NH3
(b) CH3NH2 > (CH3)2 NH > (CH3)3N > NH3
(c) NH3 > (CH3)3N > (CH3)2NH > CH3NH2
(d) (CH3)2NH > CH3NH2 > (CH3)3N > NH3
Answer/Explanation
Answer: d
Explaination:
(d) is correct 2° > 1° > 3° > NH3 because in 3° amines, there is stearic hindrance therefore, lone pair of electron is not readily available.
19. Which of the following is a 3° amine? [NCERT Exemplar]
(a) 1-methylcyclohexylamine
(b) Triethyl amine
(c) tert-butylamine
(d) N-methylaniline
Answer/Explanation
Answer: b
Explaination:
(b) Triethyl amine (C2H5)3N is tertiary amine.
20. The correct IUPAC name for CH2 = CHCH2 NHCH3 is [NCERT Exemplar]
(a) Allylmethylamine
(b) 2-amino-4-pentene
(c) 4-aminopent-l-ene
(d) N-methylprop-2-en-l-amine
Answer/Explanation
Answer: d
Explaination:
(d) Amino group is preferred over double bond.
21. Which of the following is the weakest Bronsted base? [NCERT Exemplar]

Answer/Explanation
Answer:
Explaination:
(a) Aniline is weakest because C6H5-group is electron withdrawing and there is +ve charge on 3 out of 5 resonating structures.
22. Which of the following reagents would not be a good choice for reducing an aryl nitro compound to an amine? [NCERT Exemplar]
(a) H2 (excess)/Pt
(b) LiAlH4 in ether
(c) Fe and HCl
(d) Sn and HCl
Answer/Explanation
Answer: b
Explaination:
(b) LiAlH4 is not good reducing agent to reduce nitro group.
23. The correct decreasing order of boiling points among amines and their corresponding acids and alcohols is
(a) R – CH2 NH2 > RCOOH > RCH2OH
(b) RCH2NH2 > RCH2OH > RCOOH
(c) R – CH2OH > R – CH2NH2 > RCOOH
(d) R – COOH > R – CH2OH > R – CH2NH2
Answer
Answer: d
24. Aniline is less basic than ethylamine. This is due to
(a) Conjugation of lone pair of nitrogen with the ring
(b) The insoluble nature of aniline
(c) More Kfc value of aniline
(d) Hydrogen bonding
Answer
Answer: a
25. Primary amine reacts with carbon disulphide and HgCl2 to produce alkyl isothiocyanate. This reaction is
(a) Carbylanine reaction
(b) Hoffmann bromamide reaction
(c) Perkin reaction
(d) Hoffmann mustard oil reaction
Answer
Answer: d
26. Hinsberg’s reagent is

Answer
Answer: b
27. Nitration of aniline is carried out after acylation because
(a) Acylation dectivates the — NH2 group
(b) Oxidation can be prevented
(c) O-and p-products are obtained in good yield
(d) All of these
Answer
Answer: d
28. NH2 group in aniline is
(a) Ortho directing
(b) Meta directing
(c) Ortho and para directing
(d) Para directing
Answer
Answer: c
29. Primary and secondary amines cannot be distinguished by
(a) Schiff’s reagent
(b) Carbylamine reaction
(c) Hoffmann’s bromamide reaction
(d) Iodoform test
Answer
Answer: b
30. Which of the following cannot be identified by carbyl amine test?
1. C2H5NH2
2. C6H5NH2
3. C6H5 — NH — C6H5
4. (C2H5)3N
(a) 1, 2
(b) 1, 2, 4
(c) 3, 4
(d) 2, 4
Answer
Answer: c
31.
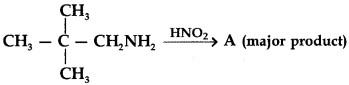
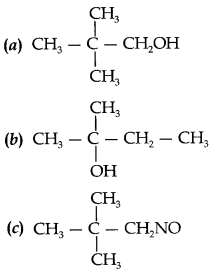

Answer
Answer: b
32.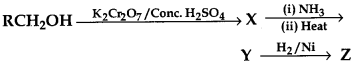
Here, Z is
(a) RCH2 CH2NH2
(b) RCH2NH2
(c) RCH2CONH2
(d) RNH2
Answer
Answer: b
Note: In the following questions two or more options may be correct. (Q.23 to Q.25)
33. Which of the following cannot be prepared by Sandmeyer’s reaction? [NCERT Exemplar]
(a) Chlorobenzene
(b) Bromobenzene
(c) Iodobenzene
(d) Fluorobenzene
Answer/Explanation
Answer:
Explaination:
(c) and (d). Iodobenzene and fluorobenzene can’t be prepared by Sandmeyer’s reaction.
34. The product of the following reaction is ____________ . [NCERT Exemplar]

Answer/Explanation
Answer:
Explaination:
(a) and (b) because -NHCOCH3 is o and -directing.
35. Under which of the following reaction conditions, aniline gives p-nitro derivative as the major product? [NCERT Exemplar]
(a) Acetyl chloride/pyridine followed by reaction with cone. H2SO4+ cone. HNO3.
(b) Acetic anyhdride/pyridine followed by cone. H2SO4 + cone. HNO3.
(c) Dil. HCl followed by reaction with cone. H2SO4 + cone. HNO3.
(d) Reaction with cone. HNO3 + cone. H2SO4.
Answer/Explanation
Answer:
Explaination:

36. Match the compounds given in Column I with the items given in Column II. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) (ii)
(b) (i)
(c) (iv)
(d) (iii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.27 and Q.28)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement. –
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
37. Assertion: Acetanilide is less basic than aniline. [NCERT Exemplar]
Reason: Acetylation of aniline results in decrease of electron density on nitrogen.
Answer/Explanation
Answer:
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
38. Assertion: Only a small amount of HCl is required in the reduction of nitro compounds with iron scrap and HCl in the presence of steam. [NCERT Exemplar]
Reason: FeCl2 formed gets hydrolysed to release HCl during the reaction.
Answer/Explanation
Answer:
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
39. The pKb of N,N-Dimethyl aniline is ____________ than aniline.
Answer/Explanation
Answer:
Explaination: lower
40. -OCH3 ____________ the basic character of amines.
Answer/Explanation
Answer:
Explaination: increases
41. CH3CH2NH2 has ____________ boiling point than (CH3)2NH.
Answer/Explanation
Answer:
Explaination: higher
42. The number of isomers of C7H9N are ____________ .
Answer/Explanation
Answer:
Explaination:

43. Tertiary amines do not react with Hinsberg’s reagent. [True/False]
Answer/Explanation
Answer:
Explaination:
True, because they do not have H attached to Nitrogen.
44. Tertiary amines form salt with HN02 soluble in water [True/False]
Answer/Explanation
Answer:
Explaination:
True. (CH3)3N + HNO2 → (CH3)3NHNO2
45. Aniline and ethyl amine can be distinguished by Azodye test. [True/False]
Answer/Explanation
Answer:
Explaination:
True. Aniline reacts with NaNO2+ HC;l at 0°C to 5°C and with phenol to give orange azodye, ethyl amine does not.
46. Give the IUPAC name of H2N—CH2—CH2—CH=CH2.
Answer/Explanation
Answer:
Explaination:
But-3-en-1 -amine.
47. What is the structure and IUPAC name of the compound, allyl amine?
Answer/Explanation
Answer:
Explaination:
![]()
48. Give the common and IUPAC name of the compound:
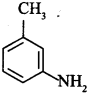
Answer/Explanation
Answer:
Explaination:
3-Methylaniline or 3-Methylbenzenamine is IUPAC name. Common name is w-Toluidine.
49. Write the chemical equations for the following chemical reactions: A primary amine is prepared from a primary alkyl halide.
Answer/Explanation
Answer:
Explaination:
RX + NH3 → RNH2 + HX
50. How is aniline obtained from benzoic acid?
Answer/Explanation
Answer:
Explaination:

51. How is /n-nitroaniline obtained from nitrobenzene? [HOTS]
Answer/Explanation
Answer:
Explaination:

52. Out of CHJNHJ and (CH3)3N, which one has higher boiling point? [Delhi 2014(C)]
Answer/Explanation
Answer:
Explaination:
CH3NH2 has higher boiling point.3
53. Arrange the following compounds in increasing order of solubility in water:
C6H5NH2, (C2H5)2NH, C2H5NH2 [Delhi 2014]
Answer/Explanation
Answer:
Explaination:
C6H5NH2 < (C2H5)2NH < C2H5NH2 is the increasing order of solubility in water.
54. Why is an alkylamine more basic than ammonia? [Delhi 2011; Foreign 2011]
Answer/Explanation
Answer:
Explaination:
It is because alkyl groups are electron releasing in alkyl amines, they will increase electron density on ‘N’, therefore, they are more basic than NH3.
55. Arrange the following compounds in an increasing order of basic strengths in their aqueous solutions:
NH3, CH3NH2, (CH3)2NH, (CH3)3N [Delhi 2013,12]
Answer/Explanation
Answer:
Explaination:
(CH3)2NH > CH3NH2 > (CH3)3N > NH3.
56. Which of the two is more basic and why? [Foreign 2014]

Answer/Explanation
Answer:
Explaination:
CH3NH2 is more basic than aniline because in CH3NH2, —CH3 group is electron releasing, which increases electron density on ‘N’, whereas in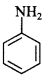 C6H5 group is electron withdrawing which decreases electron density on ‘N’.
C6H5 group is electron withdrawing which decreases electron density on ‘N’.
57. Which of the two is more basic and why? [Similar to CBSE Sample Paper 2018; Foreign 2014]

Answer/Explanation
Answer:
Explaination:
p-Toluidine is more basic than aniline because —CH3 group is electron releasing group and increases electron density on ‘N’.
58. Which of the two is more basic and why CH3NH2 or NH3? [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
CH3NH2 is more basic than NH3 because methyl group is electron releasing which increases electron density on ‘N’.
59. Arrange the following in increasing order of basic strength:
C6H5NH2, C6H5NHCH3, C6H5N(CH3)2 [Delhi 2014]
Answer/Explanation
Answer:
Explaination:
C6H5NH2 < C6H5NHCH3 < C6H5N(CH3)2 is the increasing order of basic strength because —CH3 group is electron releasing.
60. Arrange the following in increasing order of basic strength:
C6H5NH2 > C6H5NHCH3, C6H5CH2NH2 [Delhi 2014]
Answer/Explanation
Answer:
Explaination:
C6H5NH2 < C6H5NHCH3 < C6H5CH2NH2 is the increasing order of basic strength.
61. Arange in decreasing order of basic strength in gas phase:
C2H5NH2, (C2H5)2NH, (C2H5)3N, NH3
Answer/Explanation
Answer:
Explaination:
(C2H5)3N > (C2H5)2NH > C2H5NH2 > NH3
62. Arrange the following in increasing order of basic strength:
Aniline, p-Nitroaniline, p-Toludine [AI 2015(C)]
Answer/Explanation
Answer:
Explaination:
p-Nitroaniline < Aniline < p-Toludine
63. Why do amines react as nucleophiles?
Answer/Explanation
Answer:
Explaination:
It is due to presence of lone pair of electrons on nitrogen which they can donate and act as nucleophiles.
64. State the reaction taking place when: Bromine water is added to the aqueous solution of aniline. [Delhi 2016]
Answer/Explanation
Answer:
Explaination:
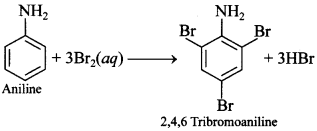
IUPAC name is 2,4,6 Tribromo benzenamine. [Delhi 2016]
65. An organic compound ‘A’ having molecular formula C2H?N on treatment with HN02 gave an oily yellow substance. Identify ‘A’. [HOTS]
Answer/Explanation
Answer:
Explaination:

66. Complete the following reaction equation: [Delhi 2015(C)]
C6H5N2CI + H3PO2 + H2O →
Answer/Explanation
Answer:
Explaination:
C6H5N2Cl + H3PO2 + H2O → C6H6 + N2 + HCl + H3PO3
67. Give a chemical test to distinguish between Aniline and Ethanamine. [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
Add NaNO2 and HCl. Cool it to 0 – 5°C, then add alkaline solution of phenol. Aniline will form orange azodye whereas ethanamine does not.
68. Write one reaction that can be used as a test for primary amines. [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
RNH2 + CHCl3 + 3KOH > R—N=ifC+ 3KCl + 3H2O is used as a test for primary amine.
Alkyl carbylamine are olfensive smelling compounds.
69. Write a chemical reaction in which the iodide ion replaces the diazonium group in a diazonium salt.
Answer/Explanation
Answer:
Explaination:
![]()
70. Give the significance of coupling reaction of aryldiazonium salts.
Answer/Explanation
Answer:
Explaination:
Coupling reaction of aryldiazonium salts with phenol or arylamines involved in the formation of azo dyes.
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 13 Amines will help you. If you have any query regarding CBSE Class 12 Chemistry Amines MCQs Pdf, drop a comment below and we will get back to you at the earliest.