Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 14 Biomolecules. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Biomolecules MCQs Pdf with Answers to know their preparation level.
Biomolecules Class 12 Chemistry MCQs Pdf
1. RNA and DNA are chiral molecules, their chirality is due to
(a) chiral bases
(b) chiral phosphate units
(c) D-sugar component
(d) L-sugar component
Answer/Explanation
Answer: c
Explaination:
(c) D-sugar component.
2. Glucose does not react with
(a) NH2OH
(b) Cone. HNO3
(c) (CH3CO)2O
(d) NaHSO3
Answer/Explanation
Answer: d
Explaination:
(d) NaHSO3 does not react with glucose.
3. The glycosidic linkage involved in linking the glucose units in amylose part of starch is
(a) C1 -C4 β-linkage
(b) C1 -C6 α-linkage
(c) C1 -C4 α-linkage
(d) C1 -C6 β-linkage
Answer/Explanation
Answer: c
Explaination:
(c) C1-C4 α-linkage are involved in α-glucose, in amylose, linear polymer or a-glucose in starch.
4. A basic amino acid among the following is
(a) glycine
(b) valine
(c) histidine
(d) leucine
Answer/Explanation
Answer: c
Explaination:
(c) It has two amino groups and one —COOH group.
5. Glucose on oxidation with Br2(aq) gives
(a) Gluconic acid
(b) Tartaric acid
(c) Sachharic acid
(d) Meso-oxalic acid
Answer/Explanation
Answer: a
Explaination:
(a) Br2/H2O oxidises —CHO to —COOH.
6. Which of the following is non-reducing sugar?
(a) Glucose
(b) Sucrose
(c) Maltose
(d) Lactose
Answer/Explanation
Answer: b
Explaination:
(b) Sucrose.
7. Which of the following statements is not correct?
(a) Ovalbumin is a simple food reserve in egg white.
(b) Blood proteins thrombin and fibrinogen are involved in blood clotting.
(c) Denaturation makes the proteins more active.
(d) Insulin maintains sugar level in the blood of a human body.
Answer/Explanation
Answer: c
Explaination:
(c) Denaturation leads to biological activity.
8. Deficiency of vitamin B, causes the disease
(a) convulsions
(b) beri beri
(c) cheilosis
(d) sterility
Answer/Explanation
Answer: b
Explaination:
(b) It is caused by deficiency of water soluble B1.
9. Which one of the following metals is required as cofactor by all enzymes utilizing ATP in phosphate transfer?
(a) K
(b) Ca
(c) He
(d) Mg
Answer/Explanation
Answer: d
Explaination:
(d) Mg acts as cofactor.
10. In aqueous solution, an amino acid exist as
(a) cation
(b) anion
(c) zwitter ion
(d) neutral molecule
Answer/Explanation
Answer: c
Explaination:
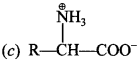
11. Glycogen is a branched chain polymer of α-D-glucose units in which chain is formed by C1—C4 glycosidic linkage whereas branching occurs by the formation of C1– C6 glycosidic linkage. Structure of glycogen is similar to __________ . [NCERT Exemplar]
(a) Amylose
(b) Amylopectin
(c) Cellulose
(d) Glucose
Answer/Explanation
Answer: b
Explaination:
(b) Glycogen is branched chain polymer of α-glucose like amylopectin.
12. Which of the following polymer is stored in the liver of animals? [NCERT Exemplar]
(a) Amylose
(b) Cellulose
(c) Amylopectin
(d) Glycogen
Answer/Explanation
Answer: d
Explaination:
(d) Glycogen is stored in liver of animals.
13. Which of the following pairs represents anomers? [NCERT Exemplar]

Answer/Explanation
Answer: c
Explaination:
(c) They differ in position of —OH group on C—1 carbon.
14. Proteins are found to have two different types of secondary structures viz. α-helix and β-pIeated sheet structure, α-helix structure of protein is stabilised by : [NCERT Exemplar]
(a) Peptide bonds
(b) van der Waals forces
(c) Hydrogen bonds
(d) Dipole-dipole interactions
Answer/Explanation
Answer: c
Explaination:
(c) H-bonds are present in α-helix.
15. Which of the following acids is a vitamin? [NCERT Exemplar]
(a) Aspartic acid
(b) Ascorbic acid
(c) Adipic acid
(d) Saccharic acid
Answer/Explanation
Answer: b
Explaination:
(b) Ascorbic acid is vitamin C, antioxidant, water soluble and its deficiency causes scurvy.
16. Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are these linkages present? [NCERT Exemplar]
(a) 5’and 3′
(b)1’and 5′
(c) 5′ and 5′
(d) 3′ and 3′
Answer/Explanation
Answer: a
Explaination:
(a) 5′ and 3′ carbon atoms.
17. Nucleic acids are the polymers of __________ . [NCERT Exemplar]
(a) Nucleosides
(6) Nucleotides
(c) Bases
(d) Sugars
Answer/Explanation
Answer: b
Explaination:
(b) Nucleic acids are polymers of nucleotides.
18. Each polypeptide in a protein has aminoacids linked with each other in a specific sequence. This sequence of amino acids is said to be __________ . [NCERT Exemplar]
(а) primary structure of proteins.
(b) secondary structure of proteins.
(c) tertiary structure of proteins.
(d) quaternary structure of proteins.
Answer/Explanation
Answer: a
Explaination:
(a) It is called primary structure having
![]()
peptide bonds.
19. Which of the following B group vitamins can be stored in our body? [NCERT Exemplar]
(a) Vitamin B1
(b) Vitamin B2
(c) Vitamin B6
(d) Vitamin B12
Answer/Explanation
Answer: d
Explaination:
(d) Vitamin B12 is stored in our body as it is neither water soluble nor fat soluble.
20. Which of the following reactions of glucose can be explained only by its cyclic structure? [NCERT Exemplar]
(a) Glucose forms pentaacetate.
(b) Glucose reacts with hydroxylamine to form an oxime.
(c) Pentaacetate of glucose does not react with hydroxylamine.
(d) Glucose is oxidised by nitric acid to gluconic acid.
Answer/Explanation
Answer: c
Explaination:
(c) It means glucose pentaacetate has cyclic structure which does not have free aldehyde group.
21. The sugar present in milk is
(a) Sucrose
(b) Maltose
(c) Glucose
(d) lactose
Answer
Answer: d
22. α-D (+) glucose and β-D (+) – glucose are
(a) Enantiomers
(b) Geometrical isomers
(c) Anomers
(d) Epimers
Answer
Answer: c
23. Distinction between glucose and fructose can be done by
(a) Benedict’s solution
(b) Tollen’s reagent
(c) Selivanoff’s reagent
(d) Fehling solution
Answer
Answer: c
24. Which does not show mutarotation?
(a) Glucose
(b) Maltose
(c) Fructose
(d) Sucrose
Answer
Answer: d
25. The reagent used for obtaining osazone derivative of fructose is
(a) NH2OH
(b) NH2 – NH2
(c) NH2 – NHC6H5
(d) 2, 4-DNP
Answer
Answer: c
26. Amylopectin is a polymer of
(a) β-D-glucose
(b) α-D-glucose
(c) β-D-frutose
(d) α-D-fructose
Answer
Answer: b
27. Hydrolysis of sucrose gives
(a) Glucose only
(b) Glucose + fructo
(c) Glucose and galactose
(d) Maltose
Answer
Answer: b
28. The disease resulting from the intake of amino acid deficient diet is
(a) Kwasiorkar
(b) Pernicicres anaemia
(c) PEM
(d) Haemophilio
Answer
Answer: a
29. Kerating present in hair is an example of
(a) Fibrous protein
(b) Globular protein
(c) Conjugated protein
(d) Derived protein
Answer
Answer: a
30. DNA and RNA differ in
(a) Sugar
(b) Purines
(c) Pyrimidines
(d) Both (a) and (c)
Answer
Answer: d
31. The vitamin present in oils and fats are
(a) A and D
(b) B and C
(c) A and B
(d) A and C
Answer
Answer: a
Note: In the following questions two or more options may be correct. (Q.21 to Q.23)
32. Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non-reducing sugar. Sucrose is a __________ . [NCERT Exemplar]
(a) monosaccharide
(b) disaccharide
(c) reducing sugar
(d) non-reducing sugar
Answer/Explanation
Answer:
Explaination:
(b) and (d). It is disachharide made up of glucose and fructose and does not have free aldehyde group, therefore, non-reducing sugar.
33. Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. Which of the following are acidic? [NCERT Exemplar]

Answer/Explanation
Answer:
Explaination:
(b) and (d) are acidic amino acids.
∵ they have 2—COOH groups and one —NH2 group.
34.
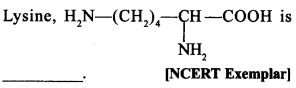
(a) α-Amino acid
(b) Basic amino acid
(c) Amino acid synthesised in body
(d) β-Amino acid
Answer/Explanation
Answer:
Explaination:
(a) and (b). It is a-amino acid.
∵ —NH2 and—COOH groups are attached to same carbon. It is basic because it has 2 amino groups and one —COOH group.
35. Match the vitamins given in Column I with the deficiency disease they cause given in Column II. [NCERT Exemplar]
| Column I (Vitamins) | Column II (Diseases) |
| (a) Vitamin A | (i) Pernicious anaemia |
| (b) Vitamin B1 | (ii) Increased blood clotting time |
| (c) Vitamin B12 | (iii) Xerophthalmia |
| (d) Vitamin C | (iv) Rickets |
| (e) Vitamin D | (v) Muscular weakness |
| (i) Vitamin E | (vi) Night blindness |
| (g) Vitamin K | (vii) Beri Beri |
| (viii) Bleeding gums | |
| (ix) Osteomalacia |
Answer/Explanation
Answer:
Explaination:
(a) (iii) and (vi)
(b) (vii)
(c) (i)
(d) (viii)
(e) (iv) and (i)
(f) (v)
(g) (ii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.25 to Q.26)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
36. Assertion: D (+) – Glucose is dextrorotatory in nature.
Reason: ‘D’ represents its dextrorotatory nature. [NCERT Exemplar]
Answer/Explanation
Answer: c
Explaination:
(c) Assertion is correct but reason is wrong statement. ‘D’ represents configuration, i.e., – OH group on right side on first chiral carbon from the bottom (+) dextrorotatory, it is also denoted by d(+).
37. Assertion: Vitamin D can be stored in our body.
Reason: Vitamin D is fat soluble vitamin. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
38. Guanine and adenine belong to __________ and Thymine and Uracil are __________ bases.
Answer/Explanation
Answer:
Explaination: purines, pyrimidines
39. Enzymes are __________ proteins.
Answer/Explanation
Answer:
Explaination: globular
40. Cellulose is linear polymer of __________.
Answer/Explanation
Answer:
Explaination: β-glucose.
41. Invert sugar is mixture of glucose and fructose and is leavorotatory. [True/False]
Answer/Explanation
Answer:
Explaination: True
42. Amylose is linear water soluble, amylopectin is water insoluble, branch chain polymer of a-glucose are two components of starch. [True/False]
Answer/Explanation
Answer:
Explaination: True
43. During denaturation of proteins, tertiary and secondary structure are ruptured but primary structure remains the same. [True/False]
Answer/Explanation
Answer:
Explaination: True
44. What are monosaccharides?
Answer/Explanation
Answer:
Explaination:
Monosaccharides are simple sugars which cannot be hydrolysed, e.g. glucose, fructose, galactose, ribose, deoxyribose, etc. are the examples of monosaccharides.
45. Name the linkage connecting monosaccha-ride units in polysaccharides.
Answer/Explanation
Answer:
Explaination: Glycosidic linkage.
46. What is the structural feature characterising reducing sugar?
Answer/Explanation
Answer:
Explaination:
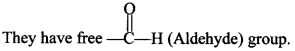
47. Give an example each of the following: [DoE]
(i) Reducing sugar
(ii) Non-reducing sugar
Answer/Explanation
Answer:
Explaination:
(i) Glucose
(ii) Sucrose
48. Under what conditions glucose is converted to gluconic acid and saccharic acid?
Answer/Explanation
Answer:
Explaination:
Bromine water converts glucose to gluconic acid, whereas cone. HNO3 converts to saccharic acid.

49. The letters ‘D’ or ‘L’ before the name of a stereoisomer of a compound indicate the correlation of configuration of that particular stereoisomer. This refers to their relation with one of the isomers of glyceraldehyde. Predict whether the following compound has ‘D’ or ‘L’ configuration.

Answer/Explanation
Answer:
Explaination:
L-configuration because —OH group is on left side on first chiral ‘C’ from bottom.
50. Why does compound (A) given below not form an oxime?

Answer/Explanation
Answer:
Explaination:
It is because it does not have free aldehyde group, therefore, it does not form oxime with hydroxyl amine (NH2OH).
51. Monosaccharides contain carbonyl group hence are classified as aldose or ketose. The number of carbon atoms present in the monosaccharide molecule are also considered for classification. In which class of monosaccharide will you place fructose ?
Answer/Explanation
Answer:
Explaination:
Fructose is keto hexose because it has 6 carbon atoms and ketone group.
52. Sucrose is dextrorotatory but the mixture obtained after hydrolysis is laevorotatory. Explain.
Answer/Explanation
Answer:
Explaination:
On hydrolysis, sucrose (dextrorotatory) gives » glucose (dextrorotatory +52.5) and fructose (laevorotatory, -92.4°). Since laevorotation of fructose is more than dextro-rotation of glucose, therefore, mixture is laevorotatory.
53. What are the products of hydrolysis of maltose? [AI 2014]
Answer/Explanation
Answer:
Explaination: 2 moles of Glucose.
54. Which disaccharide is found in milk?
Answer/Explanation
Answer:
Explaination: Lactose. It is more commonly known as milk sugar.
55. Which monosaccharide units are present in starch, cellulose and glycogen and which linkages link these units?
Answer/Explanation
Answer:
Explaination:
Starch is branched chain polymer of α-glucose, whereas cellulose is linear polymer of β-glucose. Glycogen is branched chain polymer of α-glucose.
In starch and glycogen, aα-glycosidic-linkage is present, whereas in cellulose β-glycosidic- linkage is present.
56. Which of the two components of starch is water soluble? [Delhi 2014; DoE]
Answer/Explanation
Answer:
Explaination:
Amylose is water soluble component in starch.
57. Which component of starch is a branched polymer of D-glucose and insoluble in water? [CBSE Sample Paper 2018; Delhi 2014]
Answer/Explanation
Answer:
Explaination:
Amylopectin.
58. Name the two components of D-glucose which constitute starch. [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
Amylose and amylopectin are two components of starch.
59. Why is cellulose not digested in human body?
Answer/Explanation
Answer:
Explaination:
Cellulose is not digested in human body because we do not have enzymes which can metabolise cellulose.
60. Name animal starch. Why is it called so?
Answer/Explanation
Answer:
Explaination:
Glycogen. Since its structure is similar to amylopectin and is rather more highly branched so it is also known as animal starch. It is present in liver, muscles and brain.
61. Why do amino acids show amphoteric behaviour? [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
They are basic (-NH2) as well as acidic (-COOH), therefore, amphoteric in nature.

62. Out of a, p and y amino acids, which ‘ amino acids are obtained by hydrolysis of proteins? Give its general structure.
Answer/Explanation
Answer:
Explaination:
Only a-amino acids are obtained by hydrolysis of proteins. Its general structure
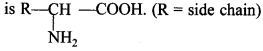
63. Give any two examples of essential amino acids.
Answer/Explanation
Answer:
Explaination: Valine and Leucine.
64. Write the name of linkage joining two amino acids. [AI 2013]
Answer/Explanation
Answer:
Explaination: Peptide linkage
65. Give an example for each of the fibrous protein and globular protein. [AI 2016]
Answer/Explanation
Answer:
Explaination:
Keratin is fibrous protein and albumin is globular protein.
66. What type of bonding occurs in P-pleated structure of proteins?
Answer/Explanation
Answer:
Explaination:
In p-pleated structure, the peptide chains are arranged side by side and these are held by a large number of intermolecular
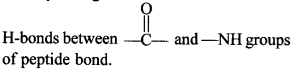
67. What is meant by tertiary structure of proteins?
Answer/Explanation
Answer:
Explaination:
Tertiary structure of proteins involves further folding and twisting of secondary structure of proteins. It has compact and folded structure. It involves H-bonding, disulphide linkage, ionic or salt bridges and hydrophobic interactions. It has van der Waals’ and electrostatic forces of attraction.
68. What are the structural changes found during denaturation of proteins?
Answer/Explanation
Answer:
Explaination:
During denaturation of proteins, 2° and 3° structures are distroyed but 1° structure remains intact.
69. What are biocatalysts? Give an example. [Foreign 2014]
Answer/Explanation
Answer:
Explaination:
Those catalysts which catalyse biochemical reactions are called biocatalysts, e.g. invertase catalyses hydrolysis of cane-sugar to form glucose and fructose.
70. Some enzymes are named after the reaction, where they are used. What name is given to the class of enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate.
Answer/Explanation
Answer:
Explaination:
Oxidoreductase.
71. How do enzymes help a substrate to be attacked by the reagent effectively?
Answer/Explanation
Answer:
Explaination:
Active site of enzyme holds the substrate molecule in suitable position so that it can be attacked by reagent effectively.
72. Out of the following groups, which group has all fat soluble vitamins:
(a) A, B-Complex, C, D
(b) A, D, E, K
(c) K, B-Complex, A, E
(d) C, A, E, D.
Answer/Explanation
Answer:
Explaination:
(b) A, D, E and K are the group of fat-soluble vitamin.
73. Name the deficiency diseases resulting from lack of vitamins A and E in the diet. [Delhi 2013(C); 2011(C); DoE]
Answer/Explanation
Answer:
Explaination:
(i) Night blindness is caused by lack of Vitamin A.
(ii) Loss of reproduction power is caused by deficiency of Vitamin E.
74. Which vitamin is linked with anti-sterility? [DoE]
Answer/Explanation
Answer:
Explaination: Vitamin E.
75. Aldopentoses named as ribose and 2-deoxyribose are found in nucleic acids. What is their relative configuration?
Answer/Explanation
Answer:
Explaination: D-configuration.
76. What are hormones? Give examples.
Answer/Explanation
Answer:
Explaination:
Hormones are chemical substances which are secreated by ductless endocrine glands and perform specific function e.g. Insulin metabolises glucose, testosterone in male, estradiol in female sex hormone.
77. What are non-steroid hormones? Name any one non-steroid hormone.
Answer/Explanation
Answer:
Explaination:
Non-steroid hormones do not contain steroid ring. An example of this type of hormone is insulin.
78. What is Thyroxine?
Answer/Explanation
Answer:
Explaination:
It is produced in thyroid gland, it is an iodine derivative of amino acid tyrosine.
79. What is cause of hypothyroidism? What are symptoms?
Answer/Explanation
Answer:
Explaination:
Low level of thyroxine is cause of hypothy-roidism, It is characterised by lethargyness and obesity. Nal should be added to common salt to prevent it.
80. What is hyperthyroidism?
Answer/Explanation
Answer:
Explaination:
Increased level of thyroxine leads to hyperthyroidism.
80. What is Addison’s disease?
Answer/Explanation
Answer:
Explaination:
If adrenal cortex does not function properly, it causes Addison’s disease, causing hypoglycemia (low level of glucose) weakness.
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 14 Biomolecules will help you. If you have any query regarding CBSE Class 12 Chemistry Biomolecules MCQs Pdf, drop a comment below and we will get back to you at the earliest.