Here we are providing Class 11 Biology Important Extra Questions and Answers Chapter 9 Biomolecules. Important Questions for Class 11 Biology are the best resource for students which helps in Class 11 board exams.
Class 11 Biology Chapter 9 Important Extra Questions Biomolecules
Biomolecules Important Extra Questions Very Short Answer Type
Question 1.
What is hydrolysis?
Answer:
During the digestion of carbohydrates, the glycosidic bond between sugar residues is broken by the addition of water and this is called hydrolysis.
Question 2.
Define fatty acid.
Answer:
Fatty acids are organic acids with a hydrocarbon chain ending in a carboxyl group.
Question 3.
What are iso-enzymes?
Answer:
The enzymes possessing slightly different molecular structures but similar in their bio-catalytic action.
Question 4.
Give the names of 2 non-polar organic solvents that are used for lipid extraction from cells.
Answer:
Chloroform, Ether.
Question 5.
Name one monosaccharide sugar that is found in the blood plasma of human beings.
Answer:
Glucose.
Question 6.
What is the function of calcium in the human body?
Answer:
Calcium in bones and teeth provides strength and rigidity to them.
Question 7.
Name the bonds uniting the monosaccharide subunits.
Answer:
Monosaccharide sub-units are joined together by glycoside bonds.
Question 8.
What are lygases?
Answer:
Lygases are the enzymes that join two substrate molecules.
Question 9.
Name the ending group of fatty acids which are organic acids with a hydrocarbon chain.
Answer:
Carboxyl.
Question 10.
Which is the most common form of sugar in fruits?
Answer:
Fructose.
Question 11.
How can we overcome the deficiency of iodine?
Answer:
By using iodized common salt.
Question 12.
Names the important food storage of carbohydrates.
Answer:
Starch and Glycogen.
Question 13.
Define allosteric modulation.
Answer:
It regulates the activity of some enzymes internally.
Question 14.
Mention two functions of the sodium and potassium ions in the body.
Answer:
Functions of the sodium and potassium ions in the body are:
- To maintain the volumes of extracellular and intracellular fluids.
- Transmission of nerve impulses.
Question 15.
Name the small molecules of the cell.
Answer:
The small molecules of the cell are:
- Minerals
- Water
- Amino acids
- Sugars
- Lipids
- Nucleotides.
Biomolecules Important Extra Questions Short Answer Type
Question 1.
What are monosaccharides? Give few examples.
Answer:
Monosaccharides are the simplest carbohydrates that cannot be hydrolyzed into still smaller carbohydrates. The general formula is Cn H2n On e.g. Ribose, Glucose, Fructose.
Question 2.
What is a disaccharide?
Answer:
A disaccharide is a sugar molecule composed of two monosaccharide sub-units e.g. a molecule of sucrose is formed from a molecule of glucose and a molecule of fructose by dehydration.
![]()
Question 3.
Why do fats release more energy than carbohydrates on oxidation?
Answer:
Like carbohydrates, fats are made up of C, H, and O but they contain fewer oxygen molecules than carbohydrates. On oxidation they consume more oxygen releasing more energy.
Question 4.
What is the function of calcium in our body? In what form is calcium deposited in the middle lamella?
Answer:
Calcium is impregnated in bones and teeth. It provides them with strength and rigidity.
Calcium is deposited in the middle lamella in the form of calcium pectate.
Question 5.
Define cellular pool. What are the characteristics of a small molecule in the cellular pool?
Answer:
The collection of various types of molecules in a cell is termed a cellular pool.
The characteristics of small molecules in the cellular pool are
- Low molecular weight
- Simple molecular conformation.
- Higher solubility.
Question 6.
What are lipids or fats? State their characteristic. What are the functions of subcutaneous fat in our body?
Answer:
Fat or lipids are esters of glycerol and fatty acids. They are made up of C, H, and O but include proportionately less oxygen as compared to carbohydrates. They are insoluble in water and soluble in non-polar organic solvents.
Functions of subcutaneous fat are
- Storage of food (chemical form of energy).
- Shock absorption.
- Insulation.
Question 7.
How are amino acids linked to form a peptide chain?
Answer:
Amino acids are condensed together to form a peptide chain. The bond is formed between the carboxyl group of one amino acid and the amino group of adjacent amino acid. This is called a peptide bond and it is formed by dehydration.
Question 8.
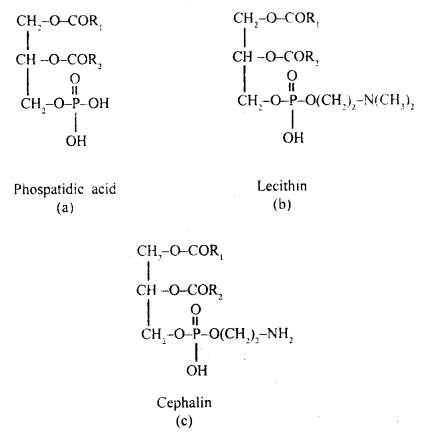
What are phospholipids?
Answer:
Phospholipids are lipids containing phosphate groups e.g. phosphoglyceride. They have a hydrophilic polar head and a hydrophobic non-polar tail.
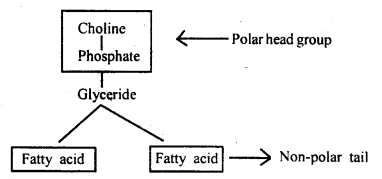
Question 9.
What are macromolecules? Give examples.
Answer:
Simple molecules assemble and form large and complex molecules called macromolecules e.g. proteins, lipids, nucleic acid, and carbohydrates.
Question 10.
Give two examples of storage polysaccharides.
Answer:
Two advantages of storing carbohydrates in the form of polysaccharides:
- Food storage polysaccharides are starch and glycogen. Starch is found in rice, wheat, etc. Glycogen is stored in the liver. During their formation, many molecules of water are removed from monosaccharides.
- If necessary, polysaccharides are broken down by enzymes for the release of energy.
Question 11.
What is chitin?
Answer:
Chitin: Chitin is similar to cellulose in many ways except its basic units are not glucose, but a similar molecule that contains nitrogen (N-acetyl glucosamine) and is soft as well as leathery.
Question 12.
Explain how glycosidic bonds are formed?
Answer:
Formation of Glycosidic Bonds: The aldehyde or ketone group of a monosaccharide can react and bind with an alcoholic group of another
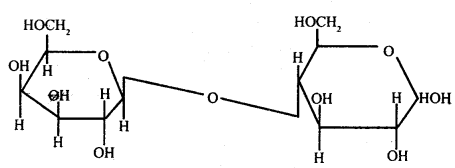
Glycosidic bond
organic compound to join the two compounds together. This bond is known as a glycosidic bond. This bond may be hydrolyzed to give the original compounds. Monosaccharides by uniting together through glycosidic bonds give rise to compound carbohydrates.
Question 13.
Describe functions of polysaccharides in living organisms.
Answer:
Food storage polysaccharides: As the name suggests saccharides which perform the function of storing food. Examples are starch, glycogen.
Starch: It is formed as a result of photosynthesis. It is found in large quantities in rice, wheat, cereals, legumes, potato, tapioca, and bananas. It is an energy-giving substance as it stores energy.
Glycogen: It is found in the muscles and liver of mammals and stores energy. There are distinct advantages of storing carbohydrates in the form of polysaccharides
- During the formation, many molecules of water get removed and bulk reduced
- Polysaccharides are relatively easy to store and get broken down easily by enzymes to release energy.
Structural Polysaccharides: Examples of these are cellulose and chitin. These take part in the formation of the organism.
Cellulose: It is a plant product. It is perhaps the most abundant material found in the living world. If forms cell walls. It is a fibrous polysaccharide that has high tensile strength. Wood and cotton have large quantities of cellulose.
Chitin: Chitin is similar to cellulose in many ways except that its basic unit is not glucose, but a similar molecule that contains nitrogen (N-acetyl glucosamine). Chitin is soft and leathery, it becomes hard when it gets impregnated with calcium carbonate or certain proteins. The insolubility of these polysaccharides in water helps in retaining the particular form it also helps in strengthening the structure of organisms.
Question 14.
What is meant by the tertiary structure of proteins?
Answer:
The tertiary structure of proteins: Many amino acid units form polypeptides. The peptide bonds holding the amino acids together in a particular way constitute the primary structure of the protein. A functional protein contains one or more polypeptide chains.
Through the formation of hydrogen bonds, peptide chains assume a secondary structure. Secondary protein may be in the form of a twisted helix or pleated sheet.
When the individual peptide chains of the secondary structure of the protein are further extensively coiled and folded into sphere-like shapes with the hydrogen bonds between the amino and carboxyl group and various other kinds of bonds cross-linking on-chain to another they form tertiary structure.
The ability of proteins to carry out specific reactions is the result of their primary, secondary, and tertiary structure.
The tertiary structure (myoglobin)
Question 15.
Explain the composition of triglycerides.
Answer:
Fat is esters of fatty acids with glycerol. Each molecule of glycerol can react with 3molecules of fatty acid. On the basis of fatty acids that are attached to the glycerol molecule, the esters are called either mono, di, or triglycerides.
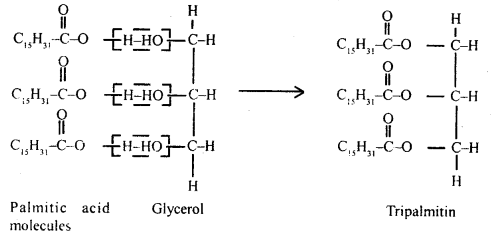
Triglyceride-Tripalmitin
Question 16.
Mention the difference between saturated and unsaturated fat?
Answer:
Differences between saturated and unsaturated fat:
| Saturated fat | Unsaturated fat |
| (i) They have higher melting points | (i) lower melting points |
| (ii) Carbon align in the chain | (ii) Double or triple banded |
| (iii) Remain in solid form at 20°c but on heating become liquids | (iii) Remain in liquid form at 20°c even in water |
Question 17.
Describe the structure of phospholipid. How are they arranged in the cell membrane?
Answer:
Structure of phospholipids: Phospholipids are a class of lipids that serve as the structural component of the cell membrane. Phospholipids have only 2 fatty acids attached to the glycerol while the 3rd glycerol binding site holds a phosphate group. This phosphate is bound to alcohol.
These lipids have both hydrophilic and hydrophobic pans due to a charge on the phosphoric acid/alcohol head of a molecule and lack a charge of the long tail of the molecule (made by fatty acids). When exposed to an aqueous solution the charged heads are attracted to the water phase and the non-polar tails are repelled from the water phase.
Phospholipids
When two layers of polar lipids come together to make a double layer, the outer hydrophilic face of every single layer will orient itself towards the solution, and the hydrophobic part will become immersed in the core of the bilayer.
Water acts as a solvent for polar molecules and the arrangement of phospholipids in the lipid bilayer of membranes is dependent on water.
Question 18.
Write short notes on
(i) Steroids
Answer:
Steroids: Steroids are complex compounds mostly found in animal hormones and cell membranes. The best example of steroids is cholesterol. The cell membrane of fungi contains ergosterol. The prostaglandins are fatty acid derivatives. They occur in minute amounts and function in blood clotting, smooth muscle contraction, and allergic reactions, etc.
(ii) Wax.
Answer:
Wax: Waxes are lipids. They are esters formed by the combination of a saturated long-chain fatty acid with long-chain alcohol. They play an important role in protection as tires form a water-proof covering over the root hairs and parts of the body in some organisms. Wax is soft and pliable. The paraffin is hard when cold. Fruits, feathers, leaves, the skin of man, and the exoskeleton of insects are waterproofed by the coating of wax. The bacteria causing TB and leprosy produce wax (Wax D.).
Question 19.
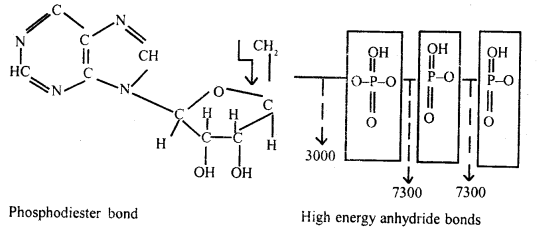
Describe the structure and function of ATP.
Answer:
ATP: It is a primary and universal carrier of chemical energy in the cell. Living cells capture, store and transport energy in a chemical form, largely as ATP and it is the ATP that is the carrier and intermediate source of chemical energy to those reactions in the cell which do not occur simultaneously.
These reactions can take place only if the chemical energy is released. It was Fritze Lipmann in 1941 who postulated this unifying concept and proposed the ATP cycle as given in the figure below.
Adenosine triphosphate
Question 20.
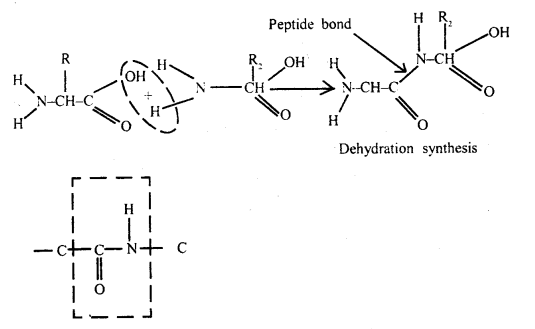
How are amino acids bonded together? Describe how these bonds are formed?
Answer:
Proteins are also formed from amino acids but they have small peptides. The two amino acids are linked by the formation of a peptide bond. Successive amino acids can be linked by peptide bonds to form a linear chain of many amino acids.
When a few amino acids are joined together, the molecule is called a peptide. Proteins are macromolecules formed from a large number of amino acids.
Peptide band
Question 21.
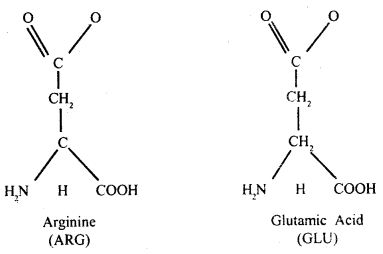
Draw the structure of amino acid.
(A) Acidic amino acid
Answer:
Amino acids: These are small molecules made of carbon, hydrogen, oxygen, and nitrogen, in certain cases sulfur is also found. Amino acids may be monocarboxylic or dicarboxylic acids bearing one or two amino groups.
The a-carbon is next to the carboxyl – C. The four valencies of the a-carbon of an amino acid hold respectively an amino (NH2) group, a carboxyl (COOH) group, a hydrogen atom, and side-chain fig (B) which may be polar or non-polar.
A free amino group is basic, a free carboxyl group is acidic. Lysine and alanine are basic amino acids because they have two amino groups and one carboxyl group. Glutamic acid and aspartic acid contain one amino and two carboxyl groups each is classified as acidic amino acids.
Alanine, glycine, valine, and phenylalanine are neutral amino acids because these contain one carboxyl group. Two amino acids can be linked by the formation Of a bond called a peptide bond. With the help of peptide bonds, many amino acids form a linear chain of many amino acids.
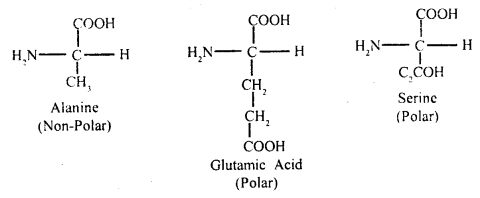
(B) Non-polar-side chain
Question 22.
Describe the primary structure of the protein.
Answer:
Primary Structure of Protein: Proteins are made of amino acids which have carboxyl group (COOH) and amino (group) (-NH2). The COOH end of an amino acid is joined to the -NH2 end of the other amino acid. Many amino acids are joined by peptide bonds which held them together in a particular sequence and constitute the primary structure of proteins. The structure does not make a protein functional.
It is a linear sequence of amino acids.
Question 23.
Name different types of RNA.
Answer:
There are three types of RNA.
- Messenger RNA (mRNA).
- Ribosomal RNA (rRNA)
- Transfer RNA (tRNA).
Question 24.
List the differences between DNA and RNA.
Answer:
Differences between DNA and RNA:
| DNA | RNA |
| (1) It consists of double-helical two polynucleotide chains. | (1) It consists of only one helical of a single polynucleotide chain. |
| (2) Deoxyribose sugar is present in the nucleotides. | (2) Rihose sugar is present in the nucleotides. |
| (3) Pyrimidine bases are thymine and cytosine. | (3) Uracil base is present instead of thymine-Cytosine is the second pyrimidine base. |
| (4) DNA synthesizes RNA to regulate cell metabolism. | (4) RNA is synthesized by DNA and carries information from DNA to regulate cell metabolism. |
| (5) DNA from the main genetic material of eukaryotes. | (5) It is the genetic material of plant viruses. |
| (6) DNA occurs in one form only. | (6) It is the genetic material of plant viruses. |
| (7) It controls the transmission of hereditary characters. | (7) It controls the synthesis of proteins in the cell. |
Question 25.
Distinguish between prosthetic group and co-factors.
Answer:
Differences between coenzyme, cofactor, and prosthetic groups:
| Cofactor | Coenzyme | Prosthetic group |
| 1. It is a non-protein substance or group that gets attached to an enzyme. | 1. It is a non-protein group that is loosely attached to the open enzyme in a functional enzyme. | 1. It is a non-protein part or group which gets attached to an open enzyme. |
| 2. It is essential for functioning. It may be organic or inorganic or metallic co-factor | 2. NAD is a coenzyme for dehydrogenases. | 2. Some prosthetic groups have metals e.g. iron porphyrin of the cytochromes. |
Question 26.
Explain the structure of an enzyme.
Answer:
Structure of an Enzyme: The enzymes are chemical substances. They catalyze the chemical reactions in the cells. They are secreted and synthesized by living cells. Most all the enzymes are proteinous in nature. Some enzymes contain a nonprotein part called the prosthetic group. Some p esthetic groups are metal compounds. NAD is a coenzyme. All enzymes have active sites.
| 1. the First carbon forms a part of the aldehyde group. | 1. Second carbon forms a part of the keto group. |
| 2. Aldoses are most commonly found in nature i.e. glucose, ribose. | 2. Ketoses are less common in nature, e.g., ribulose, fructose. |
Question 27.
Distinguish between Unsaturated fatty acids and Saturated fatty acid
Answer:
| Unsaturated fatty acid | Saturated fatty acid |
| (i) Don’t have a double band between the carbon atoms. | (i) Have one or more double bonds. |
| (ii) High melting points | (ii) Low melting points |
| (iii) Can’t be synthesized in an animal body. | (iii) Can be synthesized in the animal body |
| (iv) Don’t cause cardiovascular diseases. | (iv) Can cause cardiovascular disease |
Question 28.
Distinguish between aldose sugar and Ketose sugar
Answer:
| Aldose sugar | Ketose sugar |
| (i) First carbon forms a part of the aldehyde group | (i) Second carbon forms a part of the Keto group |
| (ii) Commonly found in nature e.g. glucose, ribose. | (ii) Less common in nature, eg. ribulose, fructose. |
Question 29.
Distinguish between Oil and Fat.
Answer:
| Oils | Fats |
| 1. Rich in unsaturated fats. | 1. Rich in saturated fats. |
| 2. Liquid at ordinary temperature. | 2. Solid or semisolid at ordinary temperature. |
| 3. Contains essential fatty acids. | 3. Do not contain essential fatty acids. |
| 4. Do not cause cardiovascular disorders e.g., vegetable oils. | 4. Can cause cardio-vascular disorders e.g., Ghee, hydrogenated vegetable oils like Dalda. |
Biomolecules Important Extra Questions Long Answer Type
Question 1.
Enlist the functions of small carbohydrates?
Answer:
- Monosaccharides are formed during the photosynthetic pathway. They are stored in plants and are utilized by other living organisms depending on them.
- Glucose is the blood sugar of many animals and on oxidation, it provides energy for all vital activities.
- Nucleotides and nucleosides contain pentose sugar in the form of ribose and deoxyribose sugars. They form a part of nucleic acids.
- Lactose of milk is formed from glucose and galactose and mammary glands of mammals.
- Glucose is used for the synthesis of fats and amino acids.
- Structural polysaccharides like cellulose and oligosaccharides are derived from mono-saccharides.
- Food storage polysaccharides like starch and glycogen are derived from monosaccharides.
Question 2.
Enumerate the functions of Lipids.
Answer:
- Lipids are storage products in plants as well as animals.
(a) In plants, fats are stored in cotyledons or endosperm to provide nourishment to the developing embryo.
(b) In animals fats are stored in adipocytes to be used whenever required by the body. - In animals, subcutaneous fats act as an insulation layer and shock \ absorber.
- They form structural components of membranes, phospholipids, glycolipids, and sterols.
- They take part in the synthesis of steroid hormones, vitamin D, and bile salts.
- Act as a solvent for fat-soluble vitamins i.e., vitamin A, D, E, and K.
- The neutral fats form a concentrated fuel producing more than twice as much energy per gram as do the carbohydrates. They thus, represent an economical food reserve in the body.
- The wax lipids form a waterproof protective coating on animal furs, plant stem, leaves, and fruits.
Question 3.
How does water help in maintaining the constancy of the internal environment of an organism?
Answer:
Some substances, capable of neutralizing acids or bases, remain in solution in the cytoplasm as extracellular fluids, e.g., bicarbonate (HCO3), carbonic acid, dibasic phosphate (HPO4-2). Acids and bases mix in the body fluids with these substances and are neutralized by them. Because of its solvent action water aids in keeping a constant pH.
Water also helps in maintaining constant body temperature by eliminating excess heat through the evaporation of sweat. Elimination of waste products through urine also helps in maintaining the constancy of the internal environment of an organism.
Question 4.
What are peroxisomes and phagosomes?
Answer:
Peroxisomes: These were for the first time observed in the kidney of rodents. They are found both in plants and animals. Their size varies from 0.5 to lp in diameter. They are delimited by a single membrane and contain a finely granular matrix. They often possess a central core called nucleoid which may consist of parallel tubules or twisted with strands. Peroxisomes are generally observed in close association with the endoplas¬mic reticulum.
Peroxisomes in different plant and animal cells differ con¬siderably in their enzymatic make-up, but they contain some peroxide-producing enzymes like urate, oxidase, D-amino acid oxidase, B-hydroxy acid oxidase, and catalase. Peroxisomes are somehow associated with some metabolic processes like photorespiration and lipid metabolism in animal cells.
Sphaerosomes: There are cell organelles bounded by a single membrane. They contain enzymes and are visible under the light microscope. These show some affinities for fat stains, including Sudan stain and sodium tetroxide.
These organelles originate from E.R. by budding. They contain enzymatic proteins which help in synthesizing oils and fats. Further devel¬opment of phagosomes takes place through an increase in the lipid content with a concomitant decrease in protein.
Question 5.
Enumerate the importance of Energy carriers.
Answer:
Energy carriers consist of nucleotides having one or two additional phosphate groups linked up at their phosphate end forming diphosphates and triphosphates. Linkage of additional phosphate groups occurs at the cost of a large amount of energy. This energy is provided by the oxidation of food mainly glucose and by photosynthesis.
Separation of the additional phosphate groups from the nucleotides by enzymatic hydrolysis releases a correspondingly large amount of energy.
Thus, ADP and ATP provide ready energy for biological activities.
The bonds joining the additional phosphate groups to the nucleotides are called high energy or energy-rich bonds, as they carry a great deal of energy. The nucleotides having more than one phosphate group are called higher nucleotides.
The energy of energy carriers, when set free is utilized for driving energy-dependent reactions in the cell and is biologically useful energy. ATP is the most common energy carrier in cells and is often called the energy currency of the cell.
Question 6.
Explain the functions of amino acids.
Answer:
- Amino acids are the building blocks for proteins.
- The amino acid Tyrosine takes part in the formation of the skin pigment melanin as well as hormones thyroxine and adrenaline.
- Glycine is important for the formation of heme.
- Tryptophan takes part in the formation of the vitamin nicotinamide.
- In plants, tryptophan forms the growth hormone indole-3- acetic acid.
- Amino acids are converted into glucose by deamination.
- Histamine and other biogenic amines are formed by the removal of carboxyl groups from amino acids.
Question 7.
Give reasons for following
(i) Salts dissolve in water but oil does not
Answer:
Water molecules are hydrogen-bonded to form short-lived macromolecular aggregates. To dissolve in water, a solute molecule must form hydrogen bonds with water molecules. Salts are polar compounds, their hydrophilic polar groups form hydrogen bonds with water molecules. So they dissolve oils having hydrophobic non-polar groups that cannot join the lattice structure of water. Thus non-polar molecules of oil do not dissolve in water.
(ii) Amino acid can be basic
Answer:
A free amino group is basic and a free carboxyl group is acidic. Amino acids can be basic because they may carry two amino groups and one carboxyl group e.g., Arginine. One free amino group causes amino acids to be basic.
(iii) Phospholipids form a thin layer on the surface of an aqueous medium.
Answer:
Phospholipids form a thin layer on the surface of an aqueous medium due to the simultaneous presence of both polar and non-polar groups in the molecule. As a result, the phospholipid molecules may arrange themselves in a double-layered membrane in aqueous media.
Question 8.
Illustrate lock and key hypothesis of enzyme action?
Answer:
Mechanism of Enzyme action: The working of enzymes is a complex one. All enzymes first of all combine with the reactions they catalyze. In other words, enzymes with substrates form an intermediate complex before decomposition of the substrate can occur.
This two-way reaction can be represented as follows.
1st step: Enzyme substrate complex = Enzyme + Product.
Formation of the enzyme-substrate complex during enzyme action.
From the above, it is clear that the enzymes must combine first with substrate molecules in order to act. In order to explain the mode of action of an enzyme. Fischer proposed a lock and key theory. According to him if the right key fits in the right lock. The lock can be opened, otherwise not.
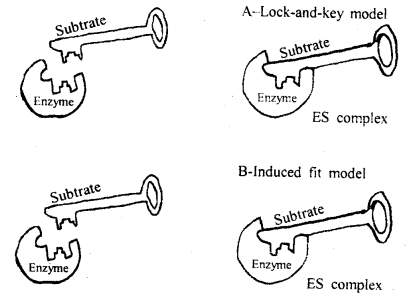
Model of enzyme activity
To explain the above in context with the enzyme action it is believed that molecules have specific configurations into which other molecules can fit. The molecules which are acted upon by the enzymes are called substrates of the enzymes. Under the above assumption, only those substrate molecules with the proper geometric shape can fit into the active site of the enzymes.
If this happens, the above molecules may compete with the substrate, and the reaction may either slow down or stop. Substances are called competitive inhibitors because they act to prevent the production of a substance.
An induced-fit model of enzyme action was given by Koshland (1959). Buttressing and catalytic are two groups of the active site of the enzyme. Their site when the substrate attaches to its bonds is broken.
Question 9.
What is the structure of DNA?
Answer:
The nucleic acids are among the largest of all molecules found in living beings. They contain three types of molecules (a) 5 carbon sugar, (b) Phosphoric acid (usually called phosphates when in chemical combi¬nation), and nitrogen-containing bases (Purines and Pyrimidines). The three join together to form a nucleotide i.e., sugar+ base + phosphate = Nucleotide. Only a few nucleotides are possible. They differ only in the kind of purines or pyrimidine (nitrogen-containing bases).
In 1953 J.D. Watson and F.H.C. Crick working in Cambridge Uni¬versity, England prepared a model of DNA molecule elucidating the struc¬ture of DNA molecule. They were awarded the Nobel Prize for this outstanding work.
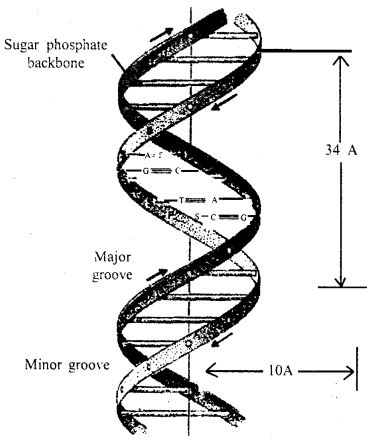
Structure of DNA
Watson and Crick model of DNA: According to Watson and Crick, the DNA molecule consisted of two strands twisted around each other in the form of a helix. Each strand is made of polynucleotides, each polynucleotide consisting of many nucleotides which remain united with its complimentary’ chain with the help of bases.
Adenine always unites with thymine and cytosine with guanine. It means that one polynucleotide chain of DNA molecule is complementary to the other.
The distance between two chains of the helix is about 20 A and the helix turns over every 34 A. Each mm of the chain consists of about 10 nucleotides.
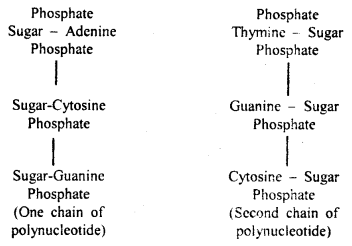
Structure of DNA
Question 10.
How does the substrate concentration affect the velocity of enzyme reaction?
Answer:
Michaelis constant or more appropriately Michaelis-Menten constant (Km) is a mathematical derivation given by Leonor Michaelis and Monde Menten in 1913 with the help of which velocity of reaction can be calculated for any substrate concentration.
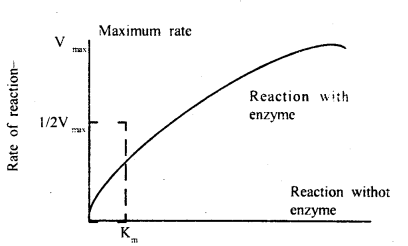
Effect of substrate concentration on enzyme action
Km or Michaelis constant is the substrate concentration at which the chemical reaction attains half its maximum velocity. The constant is an inverse measure of the affinity of an enzyme for its substrate, that is the smaller the Km the greater the substrate affinity and vice versa. The value usually lies between 104 – 105 M
Follow For More: