Here we are providing Class 11 Biology Important Extra Questions and Answers Chapter 6 Anatomy of Flowering Plants. Important Questions for Class 11 Biology are the best resource for students which helps in Class 11 board exams.
Class 11 Biology Chapter 6 Important Extra Questions Anatomy of Flowering Plants
Anatomy of Flowering Plants Important Extra Questions Very Short Answer Type
Question 1.
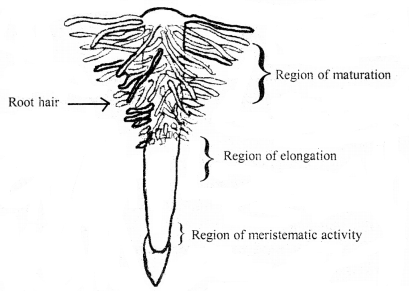
Which structure originates the lateral roots?
Answer:
Pericycle
Question 2.
What are Casparian strips?
Answer:
These are thickenings of lignin and suberin formed around the artificial walls of the endodermis to prevent plasmolysis.
Question 3.
Give an example of anomalous secondary growth.
Answer:
Bougainvillaea.
Question 4.
Name the type of wood in which vessels are absent.
Answer:
Softwood, e.g. Pinus.
Question 5.
Give an example of thick-walled parenchyma cells.
Answer:
Xylem Parenchyma in secondary tissue.
Question 6.
Which type of vascular bundles are found in Cucurbita.
Answer:
Bicollateral.
Question 7.
What are the meristematic tissues?
Answer:
They are a perpetually juvenile group of cells with the indefinite power of division.
Question 8.
What is the function of Kasparian strips?
Answer:
To prevent loss of water and minerals back to the cortex.
Question 9.
What is the function of tracheids?
Answer:
Tracheids transport water and give mechanical support to the tree.
Question 10.
What are tyloses?
Answer:
They are the vessels of hardwood containing bladder like ingrowth in the pores of lateral walls.
Question 11.
What is ring porous?
Answer:
It is a wood containing vessels of large and small sizes in distinct parts of annual rings.
Question 12.
Why do xerophytic leaves have multilayered give rise to the new plant?
Answer:
To minimize the transpiration.
Question 13.
What is diffuse-porous?
Answer:
It is a wood containing vessels of the same size in late and early.
Question 14.
What is the role of lenticel?
Answer:
It causes gaseous exchange between the atmosphere and living cells.
Question 15.
What are the functions of Tracheids?
Answer:
Tracheids transport water and give mechanical support to the tree.
Question 16.
Which tissue forms the pericycle in the dicot stem?
Answer:
Sclerenchyma tissue.
Question 17.
What is the advantage of Iigno-cellulose in the walls of the xylem?
Answer:
It provides mechanical support.
Question 18.
What uses are phloem fibres put to?
Answer:
They are used for making ropes, coarse textiles and heads.
Question 19.
Why do we notice it feasible to do grafting in monocot plants?
Answer:
Because they lack vascular cambium.
Question 20.
Name the type of plant tissue that has thin-walled cells with 12 – 140 sides and retain, the ability of division at maturity.
Answer:
Parenchyma.
Question 21.
What is phellogen?
Answer:
It is cork cambium which cuts cells upper side and lower side. The upper side cells from the phellem and lower phellogen and phelloderm constitute the periderm.
Read More:
Anatomy of Flowering Plants Biology Important Extra Questions Very Short Answer Type
Question 1.
What are tracheary elements? Of what use are these to the plants?
Answer:
These are vessels and tracheids. They are conducting cells of the xylem. The xylem vessels have perforations in their end walls while perforations are absent in tracheids, they form a continuous channel through the root, stem and leaves for the conduction of water and minerals.
Question 2.
If you are provided with microscopic preparation of transverse sections of a meristematic tissue and permanent tissue, how would you distinguish them apart?
Answer:
Meristematic tissues are composed of cells that are always in a dividing stage and divide endlessly to form new cells. These cells exist in different shapes without any intercellular spaces. These cells are thin-walled, rich in protoplasm and active with large nuclei and without vacuoles.
Permanent tissues are derived from meristematic tissue and are composed of cells, which have lost the power of division. These cells have their definite shape, size and function. These cells may be thin-walled or thick-walled.
Question 3.
What are the three basic tissues systems in Howering plants? Name the tissues under each system.
Answer:
In flowering plants, the three basic tissue systems are:
(a) DermalIt comprises the epidermis which is protective in function. During secondary growth, it is replaced by periderm.
(b) Vascular tissue system It consists of xylem and phloem and is found in the stele. In the root, the vascular bundles are renal with exarch condition whereas, in the stem, these are collateral with each condition.
(c) Ground or Fundamental Tissue It includes all the tissues except dermal and vascular, as parenchyma and sclerenchyma. It is found mainly between the epidermis and vascular cylinder and is formed of thin-walled cells with intercellular spaces in between them. Collenchyma is usually found to be thickened at the comers whereas currency nations are dead tissue and provide mechanical support.
Question 4.
Describe the structure and functions of the primary xylem.
Answer:
The primary xylem consists of tracheids vessels, xylem parenchyma and xylem fibres. The tracheids are elongated with tapering ends. They provide strength as well as help in absence of sap from roots to the leaves. The vessels are composed of a row of cells with large perforations at both ends but no bordered pits.
They help in the conduction of water and minerals and help in the storage of food. The xylem fibres are made up of sclerenchymatous cells associated with the xylem and they provide mechanical support to the plant body.
Question 5.
Why do we notice distinct rings in the tree of the temperate region while the tree of the coastal area is not distinct?
Answer:
The climatic conditions affect largely the activity of the cambium. It temperate region, the. cambium activity varies and it is not uniform throughout the year. But in coastal areas, it remains uniform throughout the year. Thus, due to the periodical activity of cambium in the temperate region, we notice distinct rings of spring and winter wood and not in the coastal area.
Question 6.
What is heartwood? Mention its any three characteristics.
Answer:
Heartwood: The hard central region of a tree trunk made up of xylem vessels that do not take part in the conduction of water.
Three characteristics of heartwood:
- It is a non-functional and dead position.
- It is dark coloured and filled with resins, tennis etc.
Question 7.
Point out the limitations of wood.
Answer:
Limitations of Wood:
- It does not change its physical and mechanical properties while heating.
- It cannot be changed into new shapes and forms.
- It is least resistant to infection caused by microorganisms and decay.
- It is combustible
Question 8.
What value is the study of plant anatomy?
Answer:
It helps to get the knowledge of plant structure and to solve various taxonomic problems. The determination of various adulterants in spices coffee, tea vegetable, dyes, tobacco saffron, as asafoetida is possible only when one knows about the anatomy of this substance.
Pharmacology and Pharmacology are dependent upon anatomical studies to know about the drug plants and their actions. It also helps in forming spurious materials from the standard woods. It also helps forensic experts fro solving criminal cases.
Question 9.
How is a cambial ring formed in dicotyledonous roots?
Answer:
Formation of the cambial ring in dicot roots: Perennial dicotyledonous root show secondary growth due to secondary meristems. Some parenchymatous cells in the phloem become meristematic. They divide and forms cambium strips there. External to the xylem a cambial ring is formed.
This lies on the inner side of the phloem cells and is cut off from the cambium on the inner and outer sides. Inner side cells form secondary xylem while the outer side cells of the cambium form secondary phloem. The primary phloem comes on the outside and the cambium forms a complete ring.
Question 10.
What are the permanent tissues?
Answer:
The permanent tissue is derived from meristematic tissues as the result of division and differentiation. They are composed of cells, which have lost the power of dividing. These cells have their definite shape, size and function. These cells may be thin-walled or thick-walled.
Question 11.
Are there any tissue elements of the xylem which are comparable to those of phloem? Comment.
Answer:
- The sieve elements of the phloem are comparable to the vessel of the xylem because both lack nucleus at maturity.
- Phloem fibre is similar to xylem fibre because both provide tensile strength to the tissue.
Phloem parenchyma and xylem parenchyma is the living components of phloem and xylem respectively.
Question 12.
Answer the following with reference to the anatomy of the monocot stem.
(i) How are the vascular bundles arranged?
Answer:
The vascular bundles are scattered in the ground tissue.
(ii) How are the xylem vessels arranged in each bundle? What do you call such an arrangement?
Answer:
Xylem vessels are ‘Y’ shaped. The big metaxylem vessels with pitted thickening from the arms, of Y. Protoxylem, occupies the lower arm of Y and is composed of spiral thickening.
But the vascular bundles are conjoint, collateral, closed and Endarch.
(iii) Vascular bundles are closed ones. What type of tissue is lacking in them?
Answer:
Cambium is absent.
Question 13.
What is phellogen? What does it produce?
Answer:
Phellogen is a cork cambium that is developed front hypodermis and epidermal cells near to the cortex. It is produced in the dicot stem during secondary growth for providing protection to the inner tissues.
It produces cork towards the outside and secondary cortex towards the inside.
Question 14.
What features make wood unique as a material?
Answer:
The following features make the wood unique as a material:
- As it is light in weight, it can be easily transported over long distances.
- It is a bad conductor of heat, electricity and sound.
- It is resistant to rust.
- It can be moulded into various desirable shapes.
- The fluctuation in temperature does not affect largely and the volume of the wood.
- Wood pulp is used for the synthesis of various materials like paper, plastics, rayon and transparent films.
Question 15.
List few important anatomical characteristics of the xerophytic leaf.
Answer:
The anatomical characteristics of the xerophytic leaf are:
- The presence of a thick cuticle on both the epidermis of the leaf offers protection and reduces the rate of transpiration.
- A multilayered epidermis is present. Its cells are elongated and lignified.
- Stomata remain sunken in the lower epidermis. This reduces the rate of transpiration.
- Palisade tissues are richly filled with chloroplast.
- It also contains crystals of calcium oxalate scattered in the upper palisade tissues of leaf cells.
Question 16.
List the differences between the internal structure of the dicot stem and the Monocot stem.
Answer:
| Dicot Stem | Monocot Stem |
| (i) Multicellular epidermal hairs are present. | (i) Epidermal hairs are generally absent. |
| (ii) Hypodermis is made up of collenchyma cells. | (ii) Hypodermis is made up of sclerenchyma cells. |
| (iii) Cortex is distinct and made up of parenchyma cells. | (iii) Cortex is not distinct but instead ground tissue is present. |
| (iv) Endodermis and pericycle are present. | (iv) Endodermis end pericycle are absent. |
| (v) A large pith is present. | (v) Pith is absent. |
| (vi) The prominent medullary ray between vascular bundles are present. | (vi) Medullary rays are absent. |
| (vii) The definite number of same size vascular bundles are arranged in a concentric ring. | (vii) The vascular bundles are of different size are found scattered in the ground tissue. |
| (viii) The vascular bundles are conjoint, collateral and open. | (viii) The vascular bundles are conjoint, collateral and closed. |
| (ix) The sheath around the vascular bundle is absent. | (ix) Bundle sheath made up of sclerenchyma cells is present around the vascular bundle. |
| (x) Lysigenous cavity is absent. | (x) Lysigenenous cavity is present in mature vascular bandies. |
| (xi) Secondary growth is present. | (xi) Secondary growth is absent. |
| (xii) Phloem parenchyma is present. | (xii) Phloem parenchyma is absent. |
Anatomy of Flowering Plants Biology Important Extra Questions Very Short Answer Type
Question 1.
What are the characters of collenchyma tissues? Give its functions also.
Answer:
Collenchyma tissue: Collenchyma tissue cells are living isodiametric without any intercellular spaces. The comer walls are thickened by Pectinisation. They appear cylindrical in vertical section and oval or polygonal in cross-section. The nucleus in each cell lies at a comer position.
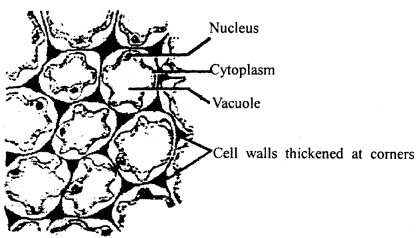
Collenchyma
They are found in the dicot stem below the epidermis and on the outer region of the leaf, midribs and pedicels. On the basis of thickening, they are of three types:
- Lamellar,
- Angular,
- Lacunate
Functions: Collenchyma tissue provides mechanical function as well v as the function of photosynthesis.
Question 2.
Draw a well-labelled diagram showing the L.S.of phloem of an angiosperm with its components.
Answer:
Phloem is a food conducting tissue and it consists of:
- Sieve, elements
- Companion cells
- Phloem fibres and
- Phloem parenchyma.
1. Sieve elements: These occur as a single cell in pteridophytes and gymnosperms and longitudinal file of cells in angiosperms. The morphological specialization of sieve plates is the development of sieve area on their walls bearing sieve plates. The sieve plate bears a large number of perforations.
The protoplasmic strands maintain continuity through these perforations within the adjoining sieve tubes. In a mature sieve element there occurs a thin layer of parietal cytoplasm and a large central vacuole. The most important features of sieve elements are that they lack a nucleus at maturity.
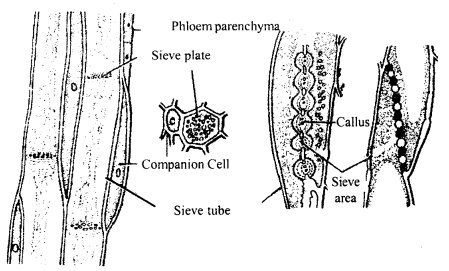
Structure of phloem (L.S)
2. Companion cells: These are thin-walled, living parenchyma narrow cells, which are closely associated with sieve tube elements. They appear rounded or polygonal with dense granular cytoplasm, « prominent nucleus and numerous small vacuoles. The companion cells lack starch.
The nuclei of the companion cells serve as the nucleus of sieve tubes as they lack them. The companion cells mainly occur in angiosperms, ac¬companying the sieve tube elements.
3. Phloem fibres: They form a prominent part of both the primary and secondary phloem. They are elongated cells with lignified walls having simple pits. They provide support and help in the transport of food material. They are used for making cords and ropes etc.
4. Phloem parenchyma: These are the living parenchyma cells associated with sieve tube cells. They are elongated with sieve tube cells. They are elongated, pointed in shape and store the starch, fat and other organic substances. The tannings and resins are also found in these cells, They are elongated like the sieve elements.
The sieve element is a living component, which lacks a nucleus at maturity.
Question 3.
Describe briefly the various types of vascular bundles.
Answer:
These are of the following types:
1. Radial The bundles in which xylem and phloem are arranged on different adulterating with each other and form the separate bundles are called radial vascular bundles as in all roots.
2. Conjoint The xylem and phloem are situated at the same radius and form a vascular bundle together.
These are divided into three types:
(a) Collateral: These are the bundles where xylem and phloem are arranged on some radius, xylem is located internally and phloem externally. These may be open when there is a patch of cambium in between the xylem and phloem e.g. Helianthus or closed when there is no cambium at all as seen in monocot stems
(b) Bicallatiral: In this vascular bundle, the phloem is found in two groups one outside the xylem elements and the other inner to them. These are always open and found in pumpkin.
(c) Concentric: The bundle in which either Phloemounds the phloem completely is known as concentric.
This exists in two forms.
- Anphicribral: The xylem lies at the centre and is surrounded by a ring of phloem, e.g., fern.
- Amphivaial: The phloem lies at the centre and is surrounded by the xylem e.g. Dracaena.
Question 4.
Describe briefly the internal structure of the monocot root with the help of a labelled diagram.
Answer:
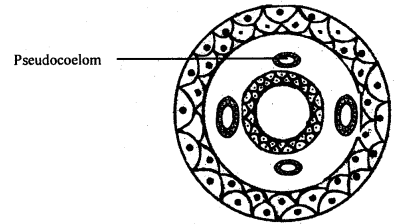
A transverse section of the monocot root shows the following issues.
- It is composed of a single layer of compactly arranged thin-walled cells without intercellular spaces and cuticle. It bears many unicellular root hair.
- Cortex: It is present beneath the epidermis. It consists of 15-20 layers of parenchymatous cells with large intercellular spaces.
- Endodermis: It is the innermost layer of the cortex. Its cells are barrel-shaped with Casparian strips on their anticlinal walls. The passage cells are seen just opposite the protoxylem ends.
- Pericycle: It consists of a single layer of thin-walled parenchymatous cells.
- Vascular bundle: The vascular bundles are radial and the xylem is exarch. The xylem and phloem bundles are always more than six.
- Pith: It occupies the central portion of the stele and is made up of parenchymatous cells.
- Conjunctive tissue: It consists of parenchymatous cells and is found between the xylem and phloem strands.
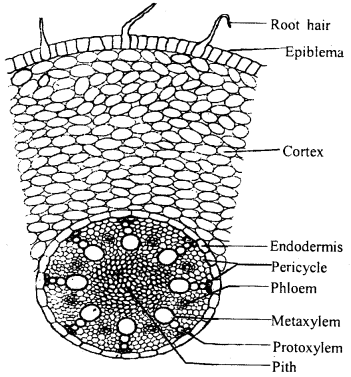
T.S. of a typical monocot root
Question 5.
Define the following.
(i) Radial vascular bundles
Answer:
Radial vascular bundles: The bundles in which xylem and phloem are arranged in different radii alternating with each other and form the separate bundles are called radial vascular bundles as in all roots.
(ii) Collateral vascular bundles
Answer:
Collateral vascular bundles: These are the bundles where xylem and phloem are arranged not at the same radius. Xylem is located internally and phloem externally. These may be open when there is a patch of cambium in between the xylem and phloem e.g. Helianthus or closed when there is no cambium at all as seen in the monocot stem.
(iii) Exarch xylem
Answer:
Exarch xylem: It is the condition where protoxylem is located towards the periphery of axis and metaxylem inwards e.g. root.
(iv) Endarch xylem
Answer:
Endarch xylem: It is the condition where metaxylem is located towards the periphery of axis and protoxylem inwards e.g. stem.
(v) Stele
Answer:
Stele: All the tissues that lie internal to Endoolerinis are collectively called stele. The outermost layer of stell is known as the pericycle.
Question 6.
Distinguish between:
(i) Phiilem and Pheiloderm
Answer:
Phiilem: It is a dead tissue that is formed by the activity of cork cambium in the outer region of the cortex during secondary growth. It is protective in function.
Pheiloderm: It is a living tissue that is formed by the activity of cork cambium in the inner side of the cortex. It regains during secondary growth. If performs the function of storage.
(ii) Open bundle and closed bundle
Answer:
Open Bundle: Avascular bundle containing cambium between xylem and phloem is called an open bundle e.g. dicot stem.
Closed Bundle: Avascular handle lacking cambium between xylem and phloem is called a closed bundle e.g. monocot stem.
(iii) Fascicular cambium and inter fascicular cambium
Answer:
Fascicular cambium: It is a strip of cambium found between the xylem and phloem of each vascular bundle of dicot stem.
Interfascicular cambium: It is a strip of cambium that is formed from the cells of medullary rays adjoining with the fascicular cambium. It occurs dining secondary growth.
(iv) Conjoint vascular bundles and Radial vascular bundles
Answer:
Conjoint vascular bundles: Xylem and phloem lie in the same bundles. They lie on different radii alternating with each other e.g. Dicot and monocot root.
Radial vascular bundles: Xylem and phloem lie in separate bundles. They lie on different radii alternating with each other e.g. Dicot and monocot root.
(v) Periderm and Bark
Answer:
Periderm: It includes three tissue consisting of phellogen, phellem and phelloderm and is formed at the peripheral region of the axis.
Bark: It includes all the tissue external to the secondary xylem formed during secondary growth. These are cambium, secondary phloem.
Must Read: