By going through these CBSE Class 11 Chemistry Notes Chapter 10 The s-Block Elements, students can recall all the concepts quickly.
The s-Block Elements Notes Class 11 Chemistry Chapter 10
→ Gp. 1 Elements: Alkaline metals Electronic configuration, Atomic & Ionic radii Ionization enthalpy, hydration enthalpy.
→ Physical & Chemical properties.
→ Uses of Alkali metals.
→ General characteristics of the compounds of alkali metals-halides, salts of oxo-acids.
→ Anomalous properties of Lithium Points of difference between Li & other alkali metals
→ Points of Similarities between Lithium & Magnesium.
→ Important compounds of Sodium: Sodium carbonate, sodium chloride, sodium hydroxide & sodium hydrogen carbonates.
→ Biological importance of sodium & potassium
→ Gp. 2 Elements: Alkaline Earth metals
→ electronic configuration, Atomic & Ionic radii, Ionization enthalpy, hydration enthalpy
→ Physical & chemical properties & use of alkaline earth metals.
→ General characteristics of compounds of alkaline earth metals- oxides & hydroxides.
→ Halides, salts of oxo-acids & carbonates.
→ Anomalous behaviour of Beryllium-Diagonal relationship between Beryllium & Aluminium.
→ Some important compounds of calcium: Calcium oxide, calcium hydroxide & calcium carbonate, calcium sulphate & cement.
→ Biological importance of Mg & ca.
→ S-block Elements: Group-1 (Alkali metals) & Group-2 (Alkaline earth metals) Their oxides & hydroxides are alkaline in nature.
→ Ionization Enthalpy: Decreases down the group.
→ Atomic & Ionic sizes: Increases down the group.
→ Diagonal Relationship: Li in group-1 & Be in group-2 shows similarities in properties to the second member of the next group. Such similarities are termed a diagonal relationship.
→ Castner-Kellner process: Sodium hydroxides are manufactured by this process.
→ Solvay process: Sodium carbonate is prepared by this process.
→ Plaster of Paris: CaSO4. \(\frac{1}{2}\) H2O
→ Portland cement: It is an important constructional material. It is manufactured by heating a pulverised mixture of limestone & clay in a rotatory kiln.
→ Importance of Sodium, Potassium, Magnesium & Calcium: Monovalent Na, K ion & divalent Mg, Ca ions are found in large proportions in Biological fluids. These ions perform important biological functions such as maintenance of unbalance & nerve impulse conduction.
“The s-block, elements are called lighter metals because of their low density.
There are two groups (1 and 2) that belong to the s-block. In these two groups of elements, the last electron enters the s-subshell of the valence shell of their atoms. They are all highly reactive metals. The elements of group 1 are called alkali metals and consist up of elements: lithium, sodium, potassium, rubidium caesium and francium. These are so-called because these metals in reaction with water form hydroxides which are strongly alkaline in nature. Their general electronic configuration is ns type.
The elements of Group 2 include beryllium, magnesium calcium, strontium, barium and radium. These elements (except beryllium) are commonly known as alkaline earth metals. These are so .called because their oxides and hydroxides are alkaline in nature and these metal oxides are found in the earth’s crust. Their general electronic configuration is ns type.
Electronic Configuration Of Alkali Metals:
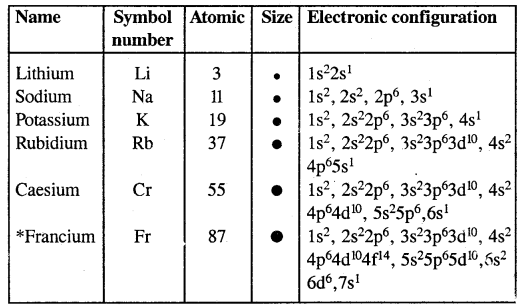
Francium is radioactive. Its largest-lived isotope 223 Fr has a half-life of only 21 minutes.
1. General characteristics of the alkali metals
(j) All the alkali metals have one valence electron ns1. This loosely held s-electron makes them the most electropositive metals which readily give M+ ions. Hence they are never found in a free state.
M → M+ + e-
Atomic and Ionic Radii
They have the largest sizes in a particular period in the periodic table. With the increase in atomic number, the atom becomes larger.
The monovalent ions (M+) are smaller than the parent atom, e.g.
Na+ → Na
K+ → K and so on
The atomic radii and ionic radii of alkali metals increase on moving down the group.
Li < Na < K < Rb < Cs and similarly
Li+ < Na+ < K+ < Rb+ < Cs+
Ionisation Enthalpies
Due to large sizes, the ionisation enthalpies of alkali metals are considerably low and decrease down the group from Li so Cs, because the effect of increasing size outweighs the increasing unclear charge.
Hydration Energy: The hydration enthalpies of alkali metal ions decrease with an increase in ionic sizes.
Li+ > Na+ > K+ > Rb+ > Cs+
Li+ ion has a maximum degree of hydration and for this reason, lithium salts are mostly hydrated e.g. LiCl.2H2O.
Physical Properties:
1. Physical Appearance: Alkali metals are silvery-white, soft and light metals.
2. Density: Because of their large size, these elements have low densities, which increases down the group from Li to Cs. However, potassium is lighter than sodium.
3. Melting points & boiling points: The melting and boiling points of the alkali metals are low indicating weak metallic bonding.
4. Flame colouration: The alkali metals and their salts impart characteristic colour to an oxidizing flame.” This is due to energy imparted to the loosely bound electron as a result of which it gets excited and jumps to higher energy levels. When the excited electron comes back to the ground state, there is the emission of radiation in the visible region.
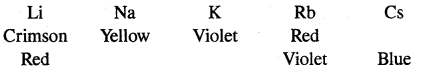
Alkali metals can therefore be detected by their flame tests.
Chemical Properties Of Alkali Metals:
The alkali metals are highly reactive due to their large size and low ionisation enthalpy and reactivity increases down the group.
1. Reactivity towards air: The alkali metals tarnish in dry air due to the formation of an oxide which in turn reacts with moisture to form hydroxides. They burn vigorously in oxygen forming oxides. Li forms monoxide, sodium forms peroxide, the other metals form superoxides.
2Li + O2 → 2LiO (Oxide)
2Na + O2 → Na2O2 (peroxide)
M + O2 → MO2 (superoxide)
[M = K, Rb, Cs]
Lithium (Li) shows exceptional behaviour in reacting with the nitrogen of air directly to form die nitride Li3N as well.
6Li + N2 → 2Li3N (from the air)
Due to extreme reactivity, these metals are kept in kerosene oil.
2. Reactivity towards water:
2M + 2H2O → 2M+ + 2OH– + H2↑
M = an alkali metal
Li reacts less vigorously with water. Other metals of the group react explosively with water. Reactivity increases down the group which is due to an increase in the electropositive character.
3. Reactivity towards hydrogen: Alkali metals react with hydrogen at about 673 K [Lithium at 1073 K] to form ionic hydrides which have high melting solids.
2M + H2 → 2M+H–
They also react with proton donors such as alcohol, gaseous ammonia and alkynes.
2C2H5OH + 2M → 2C2H5OM + H2↑
CH = CH + Na → CH ≡ C–Na+ + \(\frac{1}{2}\)H2(g)
4. Reactivity towards halogens: The alkali metals react readily with halogens to form ionic halides M+X–. However, lithium halide is somewhat covalent because of polarisation (The distortion of the electron cloud of the anion by the cation is called polarisation)
2M + X2 → 2M+X– Metallic (halide)
5. Solubility in liquid ammonia: All alkali metals are soluble in liquid ammonia. Dilute alkali metal-ammonia solution is blue in colour. With increasing concentration of metal in ammonia the blue colour starts changing to that of metallic copper after which a further amount of metal does not dissolve.
6. Reducing property (oxidation potentials): The tendency of an element to lose an electron is measured by its standard oxidation potential (E°), the more the value of E° of an element stronger will be its reducing character.
Since alkali metals have high values of E° these are powerful reducing agents and further lithium having the highest value is the strongest of them.
However, among the alkali metals, lithium although, has the highest ionization energy, yet is the strongest reducing agent. The greater reducing power of lithium is due to its larger heat of hydration which in turn is due to its small size.
7. Formation of alloys: The alkali metals form alloys amongst themselves as well as with other metals. The alkali metals dissolve readily in mercury forming amalgams. The process is highly exothermic.
General Characteristics Of The Compounds Of Alkali Metals:
(a) Oxides: Alkali metals when burnt in the air form oxides. The nature of oxides depends upon the nature of the alkali metal.
Under ordinary conditions, lithium forms the monoxide (Li2O), sodium forms the peroxide (Na2O2) and the other alkali metals form mainly superoxides (MO2) along with a small number of peroxides.
The increasing stability of the peroxide or superoxide, as the size of the metal ion increases, is due to the stabilization of large anions by larger cations through lattice energy effects. These oxides are easily hydrolysed by water to form the hydroxides according to the following reactions:
M2O + H2O → 2M+ + 2OH–
M2O2 + 2H2O → 2M+ + 2OH– + O2
2MO2 + 2H2O → 2M+ + 2OH– + H2O2 + O2
The oxides and the peroxides are colourless, but the superoxides are yellow or orange coloured. The superoxides are also paramagnetic. Sodium peroxide is widely used as an oxidizing agent in inorganic chemistry.
(b) Hydroxides: Alkali metal hydroxides, MOH are prepared, by dissolving the corresponding oxide in water. Their solubility in the water further increases as we move down the group due to a decrease in lattice energy.
Properties:
- These are white crystalline solid, highly soluble in water and alcohols. Their solubility in the water further increases as we move down the group due to a decrease in lattice energy.
- Since alkali metals are highly electropositive, their hydroxides form the strongest bases known. They dissolve in water with the evolution of much heat to give a strongly alkaline solution.
- They melt without decomposition and are good conductors of electricity in the fused state.
- These are stable to heat and do not lose water even at red heat. The thermal stability increases on moving from Li to Cs. However, they sublime at about 400°C and the vapours mainly consists of dimers. (MOH)2.
(c) Halides: Alkali metal halides arc prepared by the direct combination of the element, M and halogens. They are normally represented by the formula MX and Cs and Rb, being of large size, also form Polyhalides, i.e. Csl3
Properties:
- All alkali halides except lithium fluoride are freely soluble in water (LiF is soluble in non-polar solvents).
- They have high melting and boiling points.
- Solubility of halides of alkaline metals: The solubility of alkali metal halides show a gradation. For example
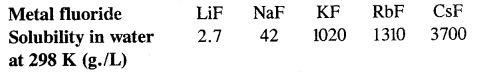
- They are good conductors of electricity infused state.
- They have an ionic crystal structure. However, lithium halides have a partly covalent character due to polarising power of Li+ ions.
(d) Carbonates and bicarbonates: All alkali metals from carbonates of the type M2CO3. Due to the high electropositive nature of the alkali metals, their carbonates (and also the bicarbonates) are highly stable to heat (however, lithium carbonate decomposes easily by heat. Further, as the electropositive character increases in moving down the group, the stability of carbonates (and bicarbonates) increases in the same order.
Both carbonates and bicarbonates are quite soluble in water and their solubility increases as we move down the group from Li to Cs. Since carbonates are salts of a weak acid (carbonic acid H2CO3), they are hydrolysed in water to give a basic solution.
2M+ + CO3– + H – OH = 2M+ + HCO32- + OH–
Since the alkali metals are highly electropositive, these are the only elements that form stable solid carbonates. However, lithium due to its less electropositive nature does not form solid bicarbonate.
(e) Hydrides: Alkaline metals form hydrides of the type M+N–. The presence of hydrogen as an anion in alkali metal hydrides is evidenced by the fact that on electrolysis hydrogen is liberated at the anode. The hydrides are not very stable. They react with water liberating hydrogen
LiH + H2O → LiOH + H2
These hydrides are, therefore, used as reducing agents. Lithium aluminium hydride, LiAlH4 is even a stronger reducing agent and is used in organic chemistry.
2. Anomalous properties of Lithium:
1. Points of difference between lithium and other Alkali Metals:
(a) Lithium is much harder, its m.p. and b.p. are higher than the other alkali metals.
(b) Lithium is the least reacting but the strongest reducing agent among all the alkali metals. On combustion in air, it forms mainly monoxide Li2O and the nitride, Li3N, unlike other alkali metals.
(c) LiCl is deliquescent and crystallizes as a hydrate, LiCl.2H2O whereas other alkali metal chlorides do not form hydrates. Lithium bicarbonate is not obtained in solid form while all other elements of this group form solid bicarbonate. Lithium unlike other alkali metals forms no acetylide on reaction with ethane.
(d) Lithium nitrate when heated gives lithium oxide Li2O whereas other alkali metal nitrates decompose to give the corresponding nitrite.
4LiNO3 → 2Li2O + 4NO2 + O2
4NaNO3 → 2NaNO2 + O2
(e) LiF and Li2O are comparatively much less soluble in water than the corresponding compounds of other alkali metals.
2. Points of similarities between Lithium and Magnesium
(a) Both lithium and magnesium are harder and lighter than other elements in their respective groups.
(b) Both Li and Mg react slowly with cold water. Their oxides and hydroxides are much less soluble and their hydroxides decompose on heating.
2LiOH → Li2O + H2O
Mg(OH)2 → MgO + H2O
(c) Both form nitrides by direct combination with N2.
6Li + N2 → 2Li3N
3Mg + N2 → Mg3N2
(d) Their oxides do not combine with an excess of O2 to give peroxide or superoxide.
(e) The carbonates of both decompose on heating to give oxide and CO2.
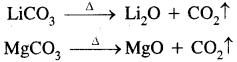
Solid bicarbonates are not formed by lithium and magnesium.
(f) Both LiCl and MgCL are soluble in ethanol.
(g) Both lithium perchlorate LiClO4 and magnesium perchlorate Mg(ClO4)2 are extremely soluble in ethanol.
(h) Both LiCl and MgCl2 are deliquescent and crystallise from aqueous solution as hydrates, LiCl.2H2O and MgCl2.8H2O.
Some Important Compounds Of Sodium:
1. Sodium carbonate (washing soda) Na2CO3.10H2O: Sodium carbonate is generally prepared by the Solvay process. In this process, the advantage is taken of the low solubility of sodium bicarbonate whereby it gets precipitated in the reaction of brine solution (sodium chloride) with ammonium bicarbonate. The latter is prepared by passing CO2 to a concentrated solution of sodium chloride saturated with ammonia.
Ammonium carbonate first formed changes to ammonium bicarbonate.
1. 2NH3 + H2O + CO2 → (NH4)2 CO3
2. (NH4)2CO3 + H2O + CO2 → 2NH4HCO3
3. NH4HCO3 + NaCl → NH4Cl + NaHCO3
Sodium bicarbonate crystal separates. These are heated to give sodium carbonate.
![]()
In this process, NH3 is recovered when the solution containing NH4Cl is treated with Ca(OH)2 Calcium chloride is obtained as a by-product.
5. 2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + H2O
Properties of sodium carbonate
- It is a white crystalline solid which exists as decahydrate, Na2CO310H2O.
- It is readily soluble in water.
- On heating, the decahydrate loses its water of crystallisation to form monohydrate. Above 373 K, the monohydrate becomes completely anhydrous and changes to a white powder called soda ash.
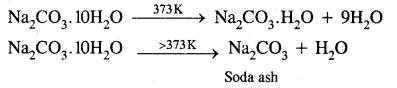
- It gets hydrolysed by water to form an alkaline solution
CO32- + H2O → HCO3– + OH–
Uses of sodium carbonate
- It is used in water-softening, laundering and cleaning.
- It is used in the manufacture of glass, soap, borax and caustic soda.
- It is used in paper, paint and textile industries.
- It is an important laboratory reagent both in qualitative and quantitative analysis.
Sodium Chloride NaCl:
Crude sodium chloride present in seawater (2.7 to 2.9% salt) is generally obtained by evaporation. It contains Na2SO4 CaSO4, CaCl2 and MgCl2 as impurities. CaCl2 and MgCl, are undesirable impurities because they are deliquescent (absorb moisture easily from the atmosphere).
To obtain pure NaCl, the crude salt is dissolved in a minimum amount of water and filtered to remove insoluble impurities. The solution is then saturated with hydrogen chloride gas. Crystals of pure sodium chloride separate out. CaCl2, and MgCl2, being more soluble than NaCl remain in the solution.
Sodium chloride melts at 1081 K. It has a solubility of 36.Ogin 100g of water at 273K. The solubility does not increase appreciably with an increase in temperature.
Sodium Hydroxide (Caustic Soda) NaOH:
It is manufactured from the electrolysis of brine solution (an aqueous solution of NaCl) by Castner-Kellner cell. A mercury cathode and carbon anode are used.
Na+Cl– (aq) → Na+(aq) + Cl– (aq)
At cathode
![]()
At anode
Cl– → \(\frac{1}{2}\) Cl2 + e–
The amalgam on treatment with water gives sodium hydroxide and H2 gas.
2Na-amalgam + 2H2O → 2NaOH + 2Hg + H2
Properties
- It is a white translucent solid.
- Its M.Pt. is 591 K.
- It gives a strongly alkaline solution in water.
- Its crystals are deliquescent.
- NaOH solution formed at the surface reacts with CO2 from the atmosphere to form a crystal of Na2CO3.
2NaOH + CO2 → Na2CO3 + H2O
Uses of sodium hydroxide:
- It is used in the manufacture of sodium metal, soap, rayon, paper, dyes and drugs.
- It is used in petroleum refining.
- Sodium hydroxide is used for mercerizing cotton to make cloth unshrinkable.
- It is used as a reagent in the laboratory.
Sodium Bicarbonate (Baking Soda) NaHCO3:
Preparation
Na2CO3 + H2O + CO2 → 2NaHCO3
Uses:
- Sodium bicarbonate is a mild antiseptic for skin infections.
- It is used in fire-extinguishers.
- It is known as baking soda because it decomposes on heating to generate bubbles of CO2 (leaving holes in cakes or pastries and making them light and fluffy).
Biological Role Of Sodium & Potassium:
K+ ions and Na+ ions are present in the red blood cells. A 70 kg weighing man contains about 90g of Na and 170gof K. Sodium ions are found primarily on the outside of cells, is located in the blood plasma and in the interstitial fluid which surrounds the cells. These ions participate in the transmission of nerve signals in regulating the flow of water across cell membranes and in the transport of sugars and amino acids into cells.
Sodium and potassium which are chemically so alike, differ quantitatively in their ability to penetrate cell membranes, in their transport mechanisms and their efficiency to activate enzymes. Thus potassium ions are the most abundant cations within cell fluids, where they activate many enzymes, participate in the oxidation of glucose to produce ATP and with sodium, are responsible for the transmission of nerve; signals.
The ionic gradients of Na+ and K+ demonstrate that a discriminatory mechanism, called the sodium-potassium pump operate across the cell membranes which consumes more than one-third of the ATP used by a resting animal- about 15 kg per 21 h in a resting human.
Group-2 Elements: Alkaline Earth Metals: The group 2 elements comprise beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), Barium (Ba) and radium (Ra). They follow alkali metals in the periodic table. These (except Beryllium) are known as alkaline earth metals.
1. Atomic properties:
(a) Electronic configuration:
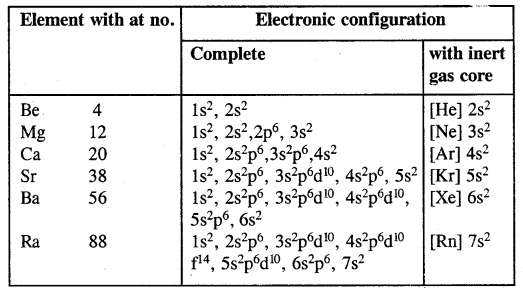
(b) Atomic and ionic sizes: The atomic and ionic radii of the alkaline earth metals are smaller than those of the alkaline metals in the corresponding periods. This is due to the increased nuclear charge in these elements.
(c) Ionization Enthalpies: The first ionization enthalpies of the alkaline earth metals are higher than those of Group 1 metals. The second ionization enthalpies of the alkaline earth metals are smaller than those of the corresponding alkali metals.
2. Physical properties of the alkaline earth metals:
(a) Physical appearance: These metals in general are silvery-white, lustrous and relatively soft, but harder than the alkali metals. Beryllium and magnesium appear to be somewhat greyish.
(b) Melting and boiling points: The fairly higher melting and boiling points of the alkaline earth metals compared to those of the corresponding alkali metals and attributed to their smaller sizes and presence of two valence electrons. The trend is, however, not systematic.
(c) Flame colour: Chlorides of alkaline earth metals, except that of Be and Mg, produce the characteristic colour of flame due to easy excitation of electrons to higher energy levels. Beryllium and magnesium atoms due to their small size, bind their electrons more strongly, i.e., their ionisation energies are high. Hence these possess high excitation energy and not excited by the energy of the flame to a higher energy state with a result no colour is produced in the flame.
(d) Electrical and thermal conductivities: These properties are characteristics of typical metals.
3. Chemical Reactivity:
(a) Action of air: Their less reactivity than the alkali metals is evident by the fact that they are only slowly oxidised on exposure to air. However, when burnt in the air, they form ionic oxides of the type MO, except Ba and Ra which give peroxides. Thus, the tendency of the metal to form higher oxides like peroxide increases on moving down the group. On ignition powdered Be burns to give BeO & Be3N2. Mg also burns with dazzling brilliance to give MgO and Mg3N2.
(b) Action of water: These metals react slowly with water liberating hydrogen and forming metal hydroxides, e.g.
Ca + 2H2O → Ca(OH)2 + H2
The reaction with water becomes increasingly vigorous on moving down the group.
Ba > Sr > Ca > Mg > Be (Reactivity with water)
The inertness of Be and Mg towards water is due to the formation of a protective thin layer of hydroxide on the surface of the metals.
(c) Action of hydrogen: All these elements, except beryllium, combine with hydrogen to form hydrides MH2, BeH2 is prepared indirectly.
2BeCl2 + LiAlH4 → 2BeH2 + LiCl + AlCl3
(d) Action of halogens: All these elements combine with halogens at elevated temperatures forming halides, MX2. Beryllium halides are covalent, while the rest are ionic. The solubility of halides (except fluoride) decreases on moving down the group.
![]()
(e) Action with nitrogen: All these elements burn in nitrogen forming nitrides, M3N2 which react with water to liberate ammonia.
3Ca + N2 → Ca3N2
Ca3N2 + 6H2O → 3Ca(OH)2 + 2NH3
The ease of formation of nitrides decreases on moving down the group.
(f) Action with acids: On account of their high oxidation potentials, they readily liberate hydrogen from dilute acids. For example.
Mg + 2HCl → MgCl2 + H2
The reactivity of alkaline earth metals increases on moving down the group. This is due to an increase in electropositive character from Be to Ba. Thus beryllium reacts very slowly, Mg reacts very rapidly while Ca, Sr and Ba react explosively.
(g) Formation of amalgam and alloys: They form an amalgam with mercury and alloys with other metals.
(h) Complex formation: Beryllium, due to its small size, forms a number of stable complexes, e.g., [BeF3]–, [BeF4]2-, [Be(H2O)]2+ etc.
(i) Reducing Character: They are strong reducing agents, Their reducing power is less than the corresponding alkali metals.
(j) Solubility in liquid ammonia: Alkaline earth metals dissolve in liquid ammonia giving coloured solutions. When the metal- ammonia solutions are evaporated, Hexammoniates M(NH3)6 are formed. The tendency for the formation of ammoniates decreases with an increase in the size of the metal atom, i.e., on moving down the group.
M + (x + y) NH3 → M(NH3)2+ + 2e– (NH3)y
4. General characteristics of compounds of the alkaline earth metals:
(a) Oxides and Hydroxides: The alkaline earth metal oxides, MO are prepared either by heating the metal in oxygen or better by calcination of carbonates.
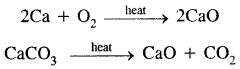
These are extremely stable, white crystalline solids. Except for BeO, all the alkaline earth oxides are ionic, in which doubly charged ions are packed in a NaCl-type of lattice leading to their high crystal lattice energy and hence high stability. However, beryllium oxide is covalent due to its small size and relatively large charge on the beryllium ion. The high melting point of BeO is due to its polymeric nature.
The heavier metal oxides react with water to form soluble hydroxides which are strong bases.
MO + H2O → M(OH)2 + heat [where M = Ca2+, Ba2+ or Sr2+)
The solubility of hydroxides of alkaline earth metals in water increases on moving down the group. This is due to the fact that with the increase in the size of the cation (down a group), the lattice energy- decreases more than the decrease in hydration energy.
Halides:
They are obtained:
- by heating the metal with halogens at high temperature or
- by treating, metal carbonates with dilute halogen acids.
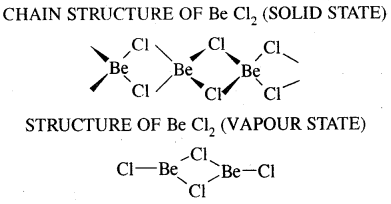
Beryllium halides are covalent compounds due to their small size and relatively high charge of Be2+ ion causing high polarising power. Due to the covalent bonding beryllium chloride, shows the following anomalous characteristics.
- It has low melting and boiling points.
- It does not conduct electricity in the fused state.
- It is soluble in organic solvents such as ether.
- It is hygroscopic and fumes in the air due to hydrolysis
BaCl2 + 2H2O → Be(OH)2 + 2HCl(g) - It is electron-deficient and behaves as Lewis acid.
The chlorides, fluorides, bromides and iodides of other alkaline earth metals are ionic solids and thus possess the following characteristics.
- The melting and boiling points are high.
- They conduct electricity in the molten state. Further, since the ionic character of the halides increases on moving down the group, the melting point and conductivity increase in the group from Mgd2 to BaCl2.
- They are “hygroscopic and readily form hydrates, e.g., MgCl2.6H2O, CaCl2.2H2O, BaCl2.2H2O.
- The halides (except fluorides) of the alkaline earth metals are soluble in water and their solubility decreases with an increasing atomic number of the metal due to a decrease in the hydration energy with the increasing size of the metal ion.
(c) Carbonates: The carbonates are invariable insoluble and therefore occur as solid rock materials in nature. However, the carbonates dissolve in water in the presence of carbon dioxide to give bicarbonates.
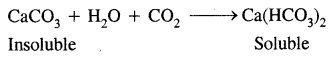
Most beryllium salts of strong oxo-acids crystallize as soluble hydrates. Beryllium carbonate is prone to hydrolysis and can be precipitated only in an atmosphere of carbon dioxide. The carbonates of magnesium and the other alkaline earth metals are all sparingly soluble in water, their thermal stability increases with increasing cationic size. Calcium carbonate finds use in the Solvay process for the manufacture of sodium carbonate in glassmaking and in cement manufacture.
(d) Sulphates: These can be prepared by dissolving the metal oxide in H2SO4.
MgO + H2SO4 → MgSO4 + H2O
The solubility of the sulphates of the alkaline earth metals decreases regularly on moving down the group. Thus beryllium sulphate is highly soluble in water, while barium and radium sulphates are practically insoluble.
The insolubility of barium sulphate is used for detecting an obstruction in the digestive system by the technique commonly known as barium meal.
The presence of BaSO4 in the stomach helps in getting X-ray pictures because of the great scattering power of heavy Ba2+ ions. Barium sulphate is also used as a white pigment.
(e) Nitrates: The nitrates are made by the dissolution of the carbonates in dilute nitric acid. Magnesium nitrate crystallizes with six molecules of water. Barium nitrate crystallizes as an anhydrous salt. All of them decompose on heating giving the oxide.
2M(NO3)2 → 2MO + 4NO2 + O2 (M = Be, Mg, Ca, Sr or Ba)
Strontium and barium nitrates are used in pyrotechnics for giving red and green flames.
Anomalous behaviour of Beryllium
The anomalous behaviour of beryllium is mainly died to its very small size and partly due to its high electronegativity. These two factors increase the polarising power [Ionic charge/ (ionic radii)2] of Be2+ ions to such extent that it becomes significantly equal to the polarising power of Al3+ ions.
Hence the two elements resemble (diagonal relationship) very much.
1. Both of them have the same value of electronegativity (1.5).
2. The standard oxidation potential of Be and Al are of the same order (Be = 1.69 V, Al = 1.7 V)
3. In nature both occur together in beryl, 3BeO, Al2O3, 6SiO2.
4. Due to its small size, beryllium has a high charge density and therefore, exhibits a strong tendency to form covalent compounds. Aluminium too has a strong tendency to form covalent compounds. Thus salts of both beryllium and aluminium have low m.p. are soluble in organic solvents and get hydrolysed by water.
Beryllium does show some tendency to form covalent compounds but other alkaline earth metals do not form covalent compounds.
5. Unlike other alkaline earth metals but like aluminium, beryllium is not easily affected by dry air.
6. Both (Be and Al) do not decompose water even on boiling; because of their weak electropositive character. Other alkaline earth » metals decompose even cold water evolving hydrogen.
7. Beryllium, like aluminium, reacts very slowly with dilute – mineral acids liberating hydrogen.
Be + 2HCl → BeCl2 + H2
2Al + 6HCl → 2AlCl3 + 3H2
Other alkaline earth metals react very readily with dilute acids.
8. The chlorides of both beryllium and aluminium have bridged chloride structures in the vapour phase.
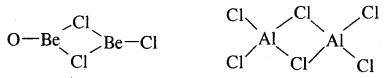
9. Salts of these, metals form hydrated ions e.g., [Be(OH2)4]3+ and [Al(OH2)6]3+ in aqueous solutions.
10. Beryllium and aluminium both react with caustic alkalies to form beryllate and aluminate respectively. Other alkaline earth metals do not react with caustic alkalies.
Some Important Compounds Of Calcium:
1. Calcium oxide (Quick lime), CaO: Preparation: By heating limestone at 1273 K
![]()
(a) The reaction is reversible and thus in order to assure the complete decomposition of CaCO3, carbon dioxide formed must be swept away by a current of air.
(b) Temperature should not be too high, because, at high temperature, clay (present as an impurity in limestone) will react with lime to form fusible silicates.
Properties:
1. Calcium oxide is a white amorphous substance.
2. When heated in an oxy-hydrogen flame, it gives an intense white light called limelight.
3. Action of water: On adding water, it gives a hissing sound and forms calcium hydroxide commonly known as slaked lime. The reaction is exothermic and known as slaking of lime.
CaO + H2O → Ca(OH)2 ΔH = – 64.5 kJ/mol
4. It reacts with SiO2 and P2O5 at high temperature forming calcium silicate, CaSiO3 and calcium phosphate; Ca3(PO4)2 respectively
6CaO + 3P2O5 → 2Ca3(PO4)2
5. With moist chlorine it forms bleaching powder, Ca(OCl)2. With moist CO2 it forms CaCO3 and with moist SO2 it forms CaSO3 and with moist HCl gas, it forms CaCl2. None of these gases will react when perfectly dried.
6. When heated with carbon at 2000°C, it forms calcium Carbide.
![]()
Uses of calcium oxide:
(a) It is used as a drying agent as such or as soda lime.
(b) Large quantities of quick-lime are used in the production of slaked lime.
(c) As a constituent of mortar, it is used on a very large scale in building constructions.
2. Calcium Hydroxide (Slaked lime), Ca(OH)2:
Preparation:
- By treating lime (quick lime) with water
Ca O + H2O → Ca(OH)2 - By the action of caustic alkalies on a soluble calcium salt.
Properties:
(a) It is a white amorphous powder, only sparingly soluble in water. Its solubility decreases with the increase in temperature.
(b) When dried and heated to redness, it loses a molecule of water and converted into calcium oxide (lime).
(c) Action of CO2: Lime water is frequently used for the detection of C02 gas. C02 gas turns lime water milky due to the formation of CaC03.
Ca(OH)2 + CO2 → CaC03(s) + H2O
However, the precipitate disappears on prolonged treatment with C02 because of the conversion of CaS03 (insoluble) to calcium bicarbonate (soluble).
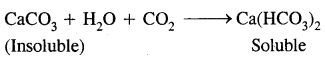
The above solution, if heated again gives turbidity. This is due to the decomposition of calcium bicarbonate to calcium carbonate,
![]()
(d) Milk of lime reacts with chlorine to form hypochlorite, a constituent of bleaching powder
2Ca(OH)2 + 2Cl2 → CaCl2 + Ca(ClO)2 + 2H2O
Uses of calcium hydroxide: Calcium hydroxide finds various uses:
(a) For absorbing acid gases
(b) For preparing ammonia from ammonium chloride
(c) In the production of mortar, a building material
(d) In glassmaking, tanning industry, for the preparation of bleaching powder and for purification of sugar.
(e) It is also used as a disinfectant
(f) As lime water in laboratories.
3. Plaster of Paris, CaSO4. \(\frac{1}{2}\) H2O
Preparation:
It is obtained when gypsum, CaSO4.2H2O is heated to 393 K
2(CaSO4.2H2O) → 2CaSO4.H2O + 3H2O
Properties:
1. It is a white powder.
2. It has a very remarkable property of setting into a hard mass on wetting with water. So, when water is added to Plaster of Paris, it sets into a hard mass in about half an hour. The setting of Plaster of Paris is due to its hydration to form crystals of gypsum which set to form a hard solid mass.
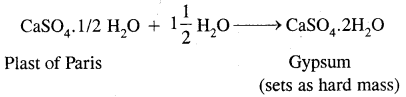
The setting of plaster of Paris is accompanied by a slight expansion in volume due to which it is used in making castes for statues, toys, etc.
Uses of Plaster of Paris:
(a) It finds extensive use in surgical bandages, in casting and moulding.
(b) It is also employed in dentistry, in ornamental work and for taking castes of statues and busts.
Properties:
(a) It is a white powder and exists in two crystalline forms: Calcite and aragonite.
(b) It is insoluble in water but dissolves in the presence of CO2 due to the formation of calcium bicarbonate.
CaCO3 + H2O + CO2 → Ca(HCO3)2
Uses:
(a) Limestone is used:
- for the manufacture of the lime, element, washing soda and glass and
- as a flux, since CaO obtained from its decomposition combines with silica to form calcium silicate, CaSiO3.
(b) Marble is used:
- for building purposes and
- in the laboratory for the production of CO2 gas.
(c) Chalk is used:
- in paints (white ash) and distempers and
- in the production of CO2 in the laboratory.
(d) Precipitated chalk is used:
- in toothpaste and powders
- in medicine for indigestion
- in adhesives and in cosmetic powders and
- to de-acidify wines.
4. Portland cement: It is made by heating a mixture of limestone (or chalk, shells etc.) with alumina silicates in carefully controlled amounts so as to give the approximate composition CaO 70%, SiO2 20%, Al2O3 5%, FeCO3 3%. The new minerals are ground to pass 300-mesh sieves and then heated in a rotary kiln to 1773 K to give sintered clinker. This is ground to 325 mesh sieve and mixed with 2-5% gypsum. An average-sized kiln can produce 1000-2000 tonnes of cement per day.
When mixed with water, the setting of cement takes place. Chemically, it is the hydration of the molecules of the constitutions and their rearrangement.
The adhesion of other particles to each other and to the embedded aggregates is responsible for the strength of the cement which is due, ultimately, to the formation of Si-O-Si-O bonds.
The purposes of adding gypsum are only to slow down the process of setting the cement so that it gets sufficiently hardened.
Concrete: It is a mixture of cement, sand, gravel (small pieces of stone) and the appropriate amount of water. When the cement concrete is filled in and around a wire-netting or skeleton of iron rods and allowed to set, the resulting structure is known as reinforced concrete (RCC).
Uses of cement: It is used in concrete and reinforces concrete, in plastering and in the construction of bridges, dams and buildings.
Biological Importance Of Magnesium & Calcium:
An adult body contains about 25g of Magnesium and 1200 g of calcium as compared with only 5g of iron and 0.06g of copper. The daily requirement in the human body has been estimated to be 200-300 mg.
All enzymes that utilize ATP in phosphate transfer require magnesium as the cofactor.
The main pigment for the absorption of light in plants for photosynthesis is green coloured chlorophyll which contains magnesium.
About 99% of body calcium is present in bones and teeth. It also plays important roles in neuromuscular function, interneuronal transmission, cell membrane integrity and blood coagulation. The calcium concentration in plasma is regulated at about 100 mg L-1. It is maintained by two hormones.
Calcitonin and parathyroid hormone. Bone is not an inert and unchanging substance but is continuously being solubilized and redeposited to the extent of 400 mg per day in man. All the calcium passes through the plasma.