By going through these CBSE Class 11 Chemistry Notes Chapter 11 The p-Block Elements, students can recall all the concepts quickly.
The p-Block Elements Notes Class 11 Chemistry Chapter 11
→ General trends in the chemistry of p-block elements.
→ Group-13 Elements: The Boron family-Electronic configuration, atomic radii, ionization, enthalpy, electro-negativity, physical & chemical properties.
→ Important trends & anomalous properties of boron
→ Important compounds of boron: Borax, orthoboric acid, diborane, uses of boron & aluminium & their compounds.
→ Group-14 Elements: The carbon family. Electronic configuration, covalent radius, ionization enthalpy, electronegativity, physical & chemical properties.
→ Important trends & anomalous behaviour of carbon.
→ Allotropes of carbon: Diamond, graphite & fullerenes & uses of carbon.
→ Important compounds of carbon & silicon: Carbon monoxide, carbon dioxide, silicon dioxide, silicones, silicates & zeolites.
→ P-BIock Elements: p-block of elements of the periodic table is unique in terms of having all types of elements-metals, non¬metals & metalloids. Group numbers ranging from 13-18.
→ Valence shell electronic configuration ns2.np1-6(Except for He).
→ pπ-pπ bonds and dπ – pπ or dπ-dπ bonds: The combined effect of size & availability of d-orbitals considerably influences the ability of these elements to form π-bonds. While the lighter elements form pπ-pπ bonds. The heavier ones form dπ-dπ bonds.
→ Electron deficiency in boron compounds: The availability of 3-valence electrons for covalent bond formation using four orbitals (2S, 2Px, 2Py & 2Pz.) leads to the so-called electron deficiency in boron compounds.
→ Boranes: Boron forms covalent molecular compounds with di-hydrogen as boranes. The simplest is diborane (B2H6).
→ Inert pair effect: Aluminium exhibits + 3 oxidation state. With heavier elements, the +1 oxidation state gets progressively stabilised on going down the group. This is a consequence of the so-called inert pair effect.
→ Catenation: The ability to form chains or rings not only with C – C single bonds but also with multiple bonds
(C = C or C ≡ C).
→ Allotropes of carbon: Three important allotropes of carbon are diamond, graphite & fullerenes.
→ Carbon monoxide: Carbon monoxide having lone pair of electrons on C forms metal carbonyls. It is deadly poisonous due to the higher stability of its haemoglobin complex as compared to that of the oxy-haemoglobin complex.
→ Carbon dioxide: Increased content of CO2 in the atmosphere due to combustion of fossil fuels & decomposition of limestone is feared to cause an increase in the greenhouse effect. This in turn raises the temperature of the atmosphere & causes serious complications.
→ Compounds of silicon: Silica, silicates & silicones are important compounds & find applications in industry & technology.
Chapter in Brief:
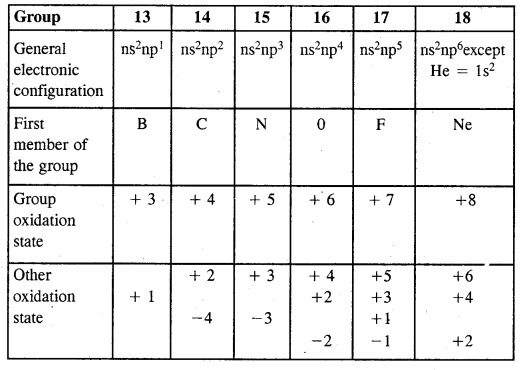
In the case of elements of p-block, the last electron enters a p-orbital. As p-subshell can hold a maximum of 6 electrons in px, py and pz atomic orbitals, p-block has 6 groups namely 13, 14, 15, 16, 17 and 18th groups. The valence shell electronic configuration is ns2 np1-6. In the case of the boron family (group 13), carbon family (group 14) and nitrogen family (group 15), the group oxidation states (the most stable oxidation states) are +3, +4 and +5 respectively for the lighter element an in the respective groups.
However, the oxidation state two until less than the group oxidation state becomes increasingly more stable for the heavier elements in each group. The occurrence of oxidation state two units less than the group’s oxidation state is due to the Inert Pair Effect.
General Electronic Configuration And Oxidation States Of P-Block Elements
Non-metals and metalloids exist only in the p-block. The non-metallic character of elements decreases down a particular group. In fact, the heaviest element in each group of the p-block is the most metallic in nature.
In general non-metals have higher ionisation enthalpies and higher electronegativities than metals. Hence in contrast to metals which readily form cations, non-metals readily form anions. The compounds formed by highly reactive non-metals like halogens with highly reactive metals like alkali metals are generally Ionic due to the large difference in their electronegativities.
On the other hand, compounds formed by non-metals themselves are largely covalent because of the small differences in their electronegativities. The change of non-metallic to metallic character can be best illustrated by the nature of oxides formed by them. The non-metallic oxides like CO2 and SiO2 are acidic or neutral whereas metallic oxides like CaO Na2O are basic.
The first member of the groups of p-block differs from the remaining members of their corresponding group in two major respects. First is the size and all other properties which depend on size. Thus, the lightest p-block elements show the same kind of differences as the lightest s-block elements, lithium and beryllium. The second important difference, which applies only to the p-block elements, arises from the effect of d-orbitals in the valence shell of heavier- elements (starting from the third period onwards) and their lack in second-period elements.
The second-period elements starting from boron are restricted to a maximum covalence of four (using 2s and three 2p orbitals). In contrast, the third-period element of a p-group with the electronic configuration 3s23pn has the vacant 3d orbitals lying between the 3p and the 4s levels of energy. Using these d-orbitals the third-period elements can expand their covalence above four. For example, while boron form only [BF4]–, aluminium gives [AlF6]3- ion. The presence of these d-orbitals influences the chemistry of the heavier elements in a number of other ways.
The combined effect of size and availability of d orbitals considerably influences the ability of these elements to form their bonds. The first member of a group differs from the heavier members in their ability to form pπ-pπ multiple bonds to itself (e.g., C = C, C ≡ C, N ≡ N) and to other second-row elements (e.g., C = 0, C = N, C ≡ N, N = 0). This type of π-bonding is not particularly strong for the heavier p-block elements. The heavier elements do form, n bonds but this involves d orbitals (dπ-pπ or dπ—dπ).
As the d orbitals are of higher energy than the p-orbitals, they contribute less to the overall stability of molecules than does pπ -pπ bonding of the second-row elements. However, the coordination number in species of heavier elements may be higher than for the first element in the same oxidation state. For example, in the +5 oxidation state both N and P form oxoanions:
NO3- (with π-bonding involving one nitrogen p-orbital ) and PO43- (four-coordination involving s,p and d orbitals contributing to the π-bonding).
Group 13 elements: The boron family
Boron, Aluminium, Gallium, Indium and Thallium are the elements present in group 13. Boron (B) is a typical non-metal. Aluminium is a metal. Gallium, indium and thallium are almost exclusively metallic in character.
Atomic & Physical Properties of Group 13 Elements
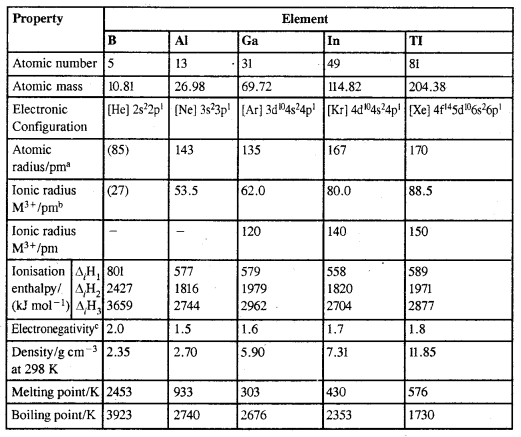

aMetallic radius, b6-coordination, cPauling scale,
For M3+ (aq) + 3e– → M(s)
eFor M+ (aq) + e– → M(s).
1. Electronic Configuration: The outer electronic configuration of these elements is ns2np1
2. Atomic Radii: Generally atomic radii increase in going down the group. However atomic radius of Ga is less than that of Al, due to the poor screening effect of the inner d-electrons for the valence electrons from the increased nuclear charge in gallium.
3. Ionisation Enthalpy: IE of Al is less than that of B due to the increased size of Al.
4. Electronegativity: Electronegativity first decreases from B to Al and then increases marginally.
5. Physical Properties: Boron is non-metallic, extremely hard and black coloured solid. It exists in many allotropic forms. It has unusually high M.Pt. The rest of the members are soft metals with low M.Pt. and high electrical conductivity Gallium with M.Pt. of 303 K is a liquid during summer. The density of elements increases down the group.
6. Chemical Properties: Due to its small size the sum of its first three enthalpies is very high. Therefore B does not form +3 cations and forms only covalent bonds. Al due to its low I.E. forms Al3+ ions. In the heavier metals due to the inert pair effect, they exhibit an oxidation state of +1.
BF3 is an electron-deficient compound and acts as a Lewis acid by accepting a pair of electrons.
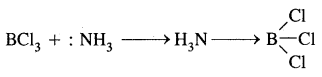
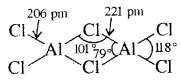
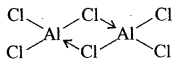
AlCl3 achieves stability by forming a dimer.
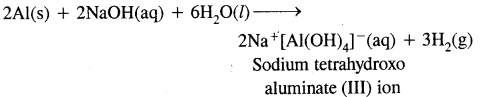
Trivalent covalent state compounds are hydrolysed by water to form tetrahedral [M(OH)4]– species, the hybridisation state of M is sp3. AlCl3 in acidified aqueous state forms octahedral [Al(H2O)6]3+ ion. Al is in d2sp3 hybridisation.
1. Reactivity towards air: Boron is unreactive in crystalline form. A1 forms a very thin oxide layer on the surface which protects the metal from further attack. On heating B2O3 and Al2O3 are formed. With N2, they form nitrides at a higher temperature.
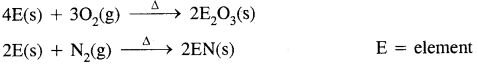
2. Reactivity towards acids and bases: B does not react. Al dissolves in dilute HCl and liberates H2 gas
2Al(s) + 6HCl(aq) → 2Al3+ (aq) + 6Cl–(aq) + 3H2(g)
Cone. HNO3 renders A1 passive by forming a protective oxide layer on the surface.
Al reacts with aq. alkalies and liberates H2 gas.
3. Reactivity towards halogens.
2E(s) + 3X2(g) → 2EX3(S) (X = F, Cl, Br, I)
E = B, Al, Ga, In.
Important Trends and Anomalous Properties of Boron:
1. The trihalides of all these elements are covalent in nature and hydrolysed by water
EX3 + 3H2O → E(OH)3 + 3HX
2. Monomeric trihalides, being electron deficient are strong LEWIS ACIDS.
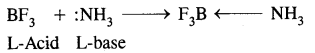
3. Maximum covalency shown by boron is 4 because it cannot expand its octet beyond 4 due to the absence of d-orbitals. Due to the availability of d-orbitals with other metals, the maximum covalent can be expected beyond 4.
AlCl3 is dimerised to AlCl6
Some Important Compounds of Boron:
1. Borax: It is a white crystalline solid of formula Na2B4O7.10H2O, more appropriately Na2[B4O5(OH)4].8H2O. It dissolves in water to give an alkaline solution.
2. Orthoboric Acid: It is a white crystalline solid with soapy touch. Its formula is H3BO3. It is sparingly soluble in water but highly soluble in hot water.
Preparation:
- Na2B4O7 (Borax) + 2HCl + 5H2O → 2NaCl + 4B(OH)3 (Boric acid)
- It is formed by hydrolysis with water of BCl3:
BCl3 + H2O(aq) → H3BO3 + 3HCl.
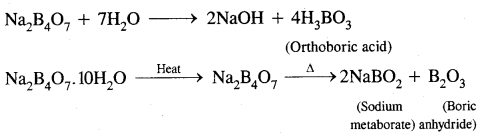
Structure: It has a layer structure in which planar BO3 units are joined by hydrogen bonds as shown in the figure below.
[Structure of boric apid H3BO3 dotted line represent hydrogen bonds.]
Properties of Boric Acid (H3BO3)
- It is a weak monobasic acid.
- It is not a protonic acid but acts as Lewis-acid by accepting electrons from a hydroxyl ion
B(OH)3 + 2HOH → [B(OH)4]- + H3O+ - On heating above 370K, metaboric acid (HBO2) is formed which on further heating yields boric oxide (B2O3).
Diborane B2H6: It is the simplest of boron hydrides.
Preparation:
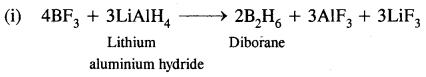
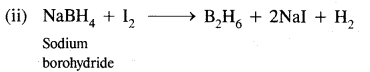
(iii) Industrially it is prepared by the reaction of BF3 on sodium hydride.
![]()
Properties of Diborane:
1. If is a colourless, highly toxic gas with a B.Pt. of 180 K.
2. It catches fire spontaneously upon exposure to air. Enormous energy is released during the reaction.
B2H6 + 3O2 → B2O3 + 3H2O; ΔCH° = -1976 kJ mol”1
3. Most of the higher boranes are highly flammable.
4. It is hydrolysed by water giving boric acid
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2O
5. Diborane undergoes cleavage reactions with Lewis bases to give borane adduct
B2H6 + 2NMe3 → 2BH3 . NMe3
B2H6 + 2CO → 2BH3 . CO
B2H6 + 2NH3 → B2H6.2NH3
which is formulated as [BH2(NH3)2]+ [NH4]– further heating gives [BH2(NH3)2]+ [BH4]– , further heating gives Borazine or Borazole or Inorganic Benzene B3N3H6
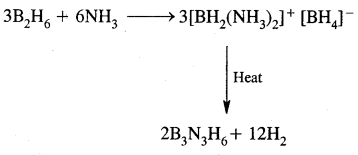
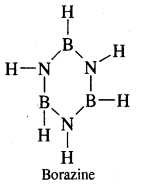
The structure of diborane is shown in Fig.(a) below. The four-terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging hydrogen atoms. The four-terminal B—H bonds are regular two centre-two-electron bonds while the two bridge (B—H – B) bonds are different and can be described in terms of three centre-two electron bonds shown in Fig.(b)
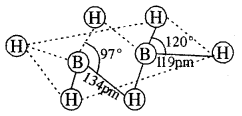
(a) The strucwre of diborane, B2H6
Boron also forms a series of Hydridoborates; the most important one is the tetrahedral [BH4]– ion. Tetrahydridoborates of several metals is known. Lithium and sodium Tetrahydridoborates is also known as Borohydrides are prepared by the reaction of metal hydrides with B2H6 in diethyl ether.
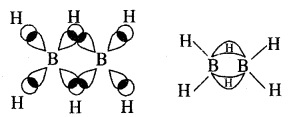
(b) Bonding in diborane. Each B atom uses sp3 hybrids for bonding.
Out of the four sp3 Iribrids on each B atom, one is without an electron shown with broken lines. The terminal B-H bonds are normal 2 centre-2 electron bonds but lie two bridge bonds are 3 centre-2 electron bonds. The 3 centres 2 electron bridge bonds are also referred to as banana bonds.
2MH + B2H6 → 2M+[BH4] ; M = Li or Na.
Both LiBH4 and NaBH4 are used as reducing agents in organic synthesis. They are starting materials for preparing other borohydrides.
Uses Of Boron & Aluminium And Their Compounds:
Boron is an extremely hard refractory solid of high melting point, low density and very low electrical conductivity find many applications. Boron fibres are used in making bullet-proof vest and light composite material for aircraft. The boron-10 (10B) isotope has a high ability to absorb neutrons and, therefore, metal borides are used in the nuclear industry as protective shields and control rods.
The main industrial application of borax and boric acid is in the manufacture of heat resistant glasses (e.g., Pyrex), glass-wool and fibreglass. Borax is also used as a flux for soldering metals, for heat, scratch and stain resistant glazed coating to earthenwares and as a constituent of medicinal soaps. An aqueous solution of orthoboric acid is generally used as a mild antiseptic.
Aluminium is a bright silvery-white metal, with high tensile strength. It has a high electrical and thermal conductivity. On a weight- to-weight basis, the electrical conductivity of aluminium is twice that of copper. Aluminium is used extensively in industry and everyday life.
It forms alloys with Cu, Mn, Mg, Si and Zn. Aluminium and its alloys can be given shapes of pipe, tubes, rods, wires, plates or foils and, therefore, find uses in packing, utensil making, construction, aeroplane and transportation industry. The use of aluminium and its compounds for domestic purposes is now reduced considerably because of its toxic nature.
Group 14 Elements: The Carbon Family
Carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb) are the members of group 14.
1. The valence shell electronic configuration of these elements is ns2np1.
2. Covalent Radius: There is a considerable increase in covalent radius from C to Si, thereafter from Si to Pb, a small increase in radius is observed. This is due to the presence of completely filled d and f-orbitals in heavier members.
Atomic And Physical Properties Of Group 14 Elements:
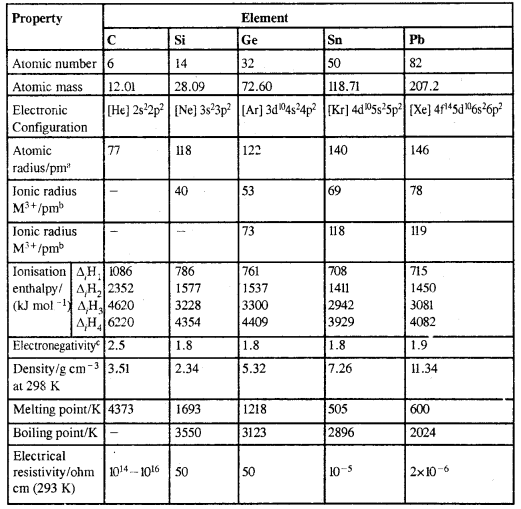
afor MIV oxidation state; b6-coordination, cPauling scale, d293 K; efor diamond; for graphite, density is 2.22; fβ-form (stable at room temperature)
3. Ionization Enthalpy: The first TE of group 14 members is higher than the corresponding members of group 13. It generally decreases from top to bottom. There is a small increase in the case of lead and it is due to the poor shielding effect of intervening d and f orbitals and the increase in the size of the atom.
4. Electronegativity: Due to the small size, the elements of this group are slightly more electronegative than group 13 elements. The electronegativity values for elements from Si to Pb are almost the same.
5. Physical Properties: All group 14 elements are solids, C and Si are non-metals, germanium (Ge) is a metalloid, whereas tin and lead are soft metals with low melting points. Melting points and boiling points of group 14 elements are much higher than those of the corresponding elements of the. group 13 elements.
6. Chemical Properties:
Oxidation states and trends in chemical reactivity: The common oxidation states shown by these elements are +4 and +2. Since the sum of four ionisation enthalpies is very high, compounds in the +4 oxidation state are generally covalent. The heavier members Ge, Sn and Pb, tendency to show an oxidation state of +2 increases due to the inert pair effect, i.e., the two electrons in ns2 orbital prefer to remain paired if we go down the group and do not participate in bond formation.
C & Si mostly show an oxidation state of + 4.
Ge shows a + 4 states in stable compounds and only a few compounds in a + 2 oxidation state.
Sn forms compounds in both oxidation state + 4 and + 2 (Sn in + 2 states is a reducing agent)
Lead compounds in the + 2 state are stable and in the + 4 states are strong oxidising agents.
Being electron-precise molecules, they are neither electron- acceptors nor electron-donors.
Although C cannot expand its octet beyond 4 due to the non-availability of d-orbitals, other elements of the group can do so, because of the presence of d-orbitals in them. CCl4 can’t undergo hydrolysis, whereas SiCl4 can do so due to the same reason.
For examples, the species like SiF5–, SiF62-, GeCl62- and [Sn(OH)6]2- exist where the hybridisation of the central atom is sp2d3.
1. Reactivity towards oxygen: All members on heating in oxygen form oxides-MO and MO2. Oxides in a higher oxidation state are more acidic than in a lower oxidation state. CO is neutral, CO is acidic. The dioxides-CO2, SiO2, GeO2 are acidic, SnO2 and PbO2 are amphoteric. GeO is distinctly acidic, SnO and PbO are amphoteric.
2. Reactivity towards water:
C, Si, and Ge are not affected by water
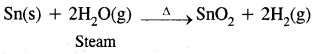
Pb is not affected by water.
3. Reactivity towards halogens
M + X2 → MX2 M: Si, Ge, Sn, Pb
M + 2X2 → MX4 X: F, Cl, Br, I
Most MX4 are covalent M shows sp3 hybridisation and MX4 are tetrahedral in shape. SnF4 & PbF4 are ionic in nature. Pbl4 does not exist. Stability of MX2 increases down the group.
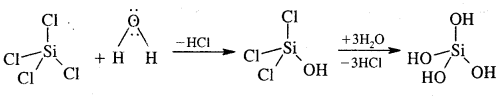
GeX4 is more stable than GeX2, whereas PbX2 is more stable than PbX4. Sid4 undergoes hydrolysis as shown below, but CCl4 cannot undergo hydrolysis because carbon cannot expand its covalence beyond four due to the absence of d-orbitals
Important Trends and Anomalous Behaviour of C:
Carbon (C) the first member of group 14 differs from its congeners due to
- Small size
- Higher ionisation enthalpy and higher electronegativity.
- Non-availability of d-orbitals.
In carbon, only s and p orbitals are available for bonding and, therefore, it can accommodate only four pairs of electrons around it. This would limit the maximum covalence to four whereas other members can expand their covalence due to the presence of d orbital.
Carbon also has a unique ability to form pπ-pπ multiple bonds with itself and with other atoms of small size and high electronegativity. Few examples of multiple bonding are: C = C, C ≡ C, C=0, C = S, and C = N. Heavier elements do not form pπ-pπ bonds because their atomic orbitals are too large and diffuse to have effective overlapping.
Carbon atoms have the tendency to link with one another through covalent bonds to form chain and rings. This property is called catenation. This is because C—C bonds are very strong. Down the group the size increases and electronegativity decreases, and, thereby, the tendency to show catenation decreases. This can be clearly seen from bond enthalpies values. The order of catenation is C >> Si > Ge = Sn. Lead does not show catenation.
| Bond | Bond enthalpy/kJ mol-1 |
| C-C | 348 |
| Si-Si | 297 |
| Ge-Ge | 260 |
| Sn-Sn | 240 |
Due to the property of catenation and pπ-pπ bonds formation, carbon is able to show allotropic forms.
Allotropes of Carbon:
Carbon exists in crystalline and amorphous forms. Diamond and graphite are two well-known crystalline forms of carbon. In 1985, the third form of C known as Fullerenes was discovered:
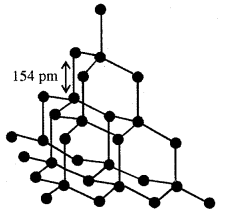
The structure of diamond
Carbon in diamond is sp3 hybridised. Diamond has a crystal lattice. The C—C bond length is 154 pm. The structure is a rigid three-dimensional network of carbon atoms. In this structure shown on the side, directional covalent bonds are present throughout the lattice.
It is very difficult to break extended covalent bonding and therefore diamond is the hardest substance on the earth. It is used as an abrasive for sharpening hand tools, in making dies and in the manufacture of tungsten filaments for electric light bulbs.
Graphite:
Graphite has a layered structure. Layers are held by van der Waals forces and the distance between the two layers is 340 pm. Each layer is composed of planar hexagonal rings of C atoms. C—C bond length within a layer is 142 pm. Here C undergoes sp2 hybridisation and makes three bonds with 3 neighbouring C atoms. The fourth electron forms a bond. The electrons are delocalised over the whole sheet.
These electrons in graphite are mobile and therefore, graphite conducts electricity- Graphite is very soft and is used as a dry lubricant in machines running at high temperature, where oil cannot be used as a lubricant.
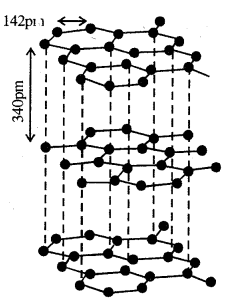
Structure of graphite
Fullerenes:
Fullerenes are made by heating graphite in an electric arc in the presence of an inert gas such as helium or argon. The sooty material formed by the condensation of vapourised C60 small molecules consists up mainly of a smaller quantity of C70 and traces of fullerenes consisting of an even number of carbon atoms up to 350 or above. Fullerenes are the only pure forms of carbon because they have smooth structure without having “dangling” bonds.
Fullerenes are cage-like molecules. the molecule has a shape like a soccer ball and is called Buckminster Fullerene. It contains twenty six-membered rings and twelve five-membered rings. A six-membered ring is fused with six or five-membered rings but a five-membered ring can only fuse with six-membered rings.
All the carbons atoms are equal and they undergo sp3 hybridisation. Each carbon atom forms three sigma bonds with the other three carbon atoms. The remaining electron at each carbon atom is delocalised in molecular orbitals which give an aromatic character to the molecule.
This ball-shaped molecule has 60 vertices and each one is occupied by one C atom and it contains both single and double bonds with C-C distances of 143.5 pm and 138.3 pm respectively. Spherical fullerenes are also called Bucky Balls
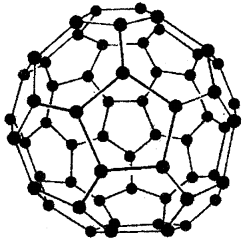
The structure of C 60, Buckminster fullerene. Note that molecule has the shape of a soccer ball (football)
Graphite is a thermodynamically most stable allotrope of carbon and therefore ΔfH° of graphite is taken as zero.
Uses of Carbon:
- Graphite fibres embedded in plastic material form high strength, lightweight composites which find wide applications.
- Being a good conductor, graphite is used as electrodes in batteries and in industrial electrolysis.
- Crucibles made of graphite are inert to dilute acids and alkalies.
- Graphite is used as a moderator in nuclear reactors to slow down the speed of fast-moving neutrons.
- Being highly porous activated charcoal is used in absorbing poisonous gases. It is also used in water filters to remove organic contaminators and in the air conditioning system to control odour.
- Carbon black is used as a black pigment in black ink and as filler in automobile tyres.
- Coke is used as a fuel and largely as a reducing agent in metallurgy.
- Diamond is a precious stone and used in jewellery. It is measured in carat (1 carat = 200 mg)
Some Important Compounds of Carbon and Silicon:
- Oxides of Carbon: Two important oxides of C are carbon monoxide CO and carbon dioxide CO2.
- Carbon Monoxide (CO): Direct oxidation of carbon in a limited supply of air or oxygen yields CO.
1. Lab. method: On a small scale CO is prepared by dehydration of formic acid with cone. H2SO4 at 373K
![]()
2. Commercial-scale: It is prepared commercially by the passage of steam over hot coke. The mixture of CO and H2 produced is called water-gas or synthesis gas
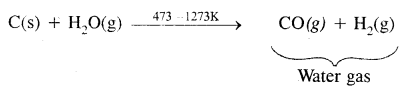
When air is used instead of steam a mixture of CO and N2 produced which is called producer gas.
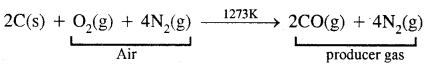
Properties:
- It is a colourless odourless gas.
- It is almost insoluble in water;
- It- is a powerful reducing agent and reduces all metal oxides other than those of alkali and alkaline earth metals, aluminium and a few transition elements.
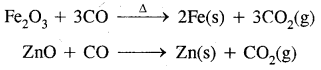
- In C ≡ O: there is one sigma and two n bonds between C and oxygen. Because of the presence of a lone pair of electrons on C, the CO molecule acts as a donor and reacts with certain metals when heated to form metal carbonyls.
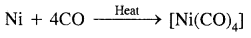
- Due to its highly poisonous nature, CO forms a complex with haemoglobin which is about 300 times more stable than the oxygen complex. This prevents haemoglobin in the red blood corpuscles from carrying oxygen around the body and ultimately results in death.
Carbon Dioxide:
Methods of Preparation.
- Complete combustion of C and C containing fuels.
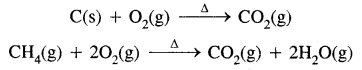
- Lab. method
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l) - Commercially, it is prepared by heating lime stone,
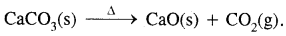
Properties:
- It is colourless and odourless gas.
- It has low solubility in water. With water, it forms carbonic acid H2CO3 which is a weak dibasic acid.
H2CO3– + H2O ⇌ HCO3– + H3O+
HCO3– + H2O ⇌ CO32- + H3O+ - 2NaOH + CO2 → Na2CO3 + H2O
- Photosynthesis
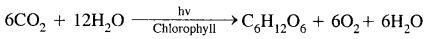
- Excess of CO2 in the atmosphere leads to the greenhouse effect which will raise the temperature of the atmosphere.
- CO2 in the solid state is called Dry ice which is used as a refrigerant for ice cream and frozen food.
Structure of CO2
C in CO2 undergoes sp hybridisation. Two sp hybridised orbitals of carbon atom overlap with two p orbitals of oxygen atoms to make two sigma bonds while the other two electrons of the carbon atom are involved in pπ-pπ bonding with an oxygen atom. This results in its linear shape [with both C-O bonds of equal length (115 pm)] with no dipole moment. The resonance structures are shown below:
![]()
Resonance structures of carbon dioxide
Silicon Dioxide SiO2
Silicon dioxide or silica along with silicates constitute 95 % of the earth’s crust. SiO2 is a covalent three-dimensional network solid in which each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Each oxygen atom in turn covalently bonded to another silicon atoms as shown.
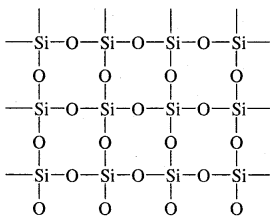
Three-dimensional structure of SiO
Properties:
- Silica in its normal state is almost non-reactive.
- It is attacked by HF and NaOH.
SiO2 + 4HF → SiF4 + 2H2O
SiO2 + 2NaOH → Na2SiO3 (Sodium silicate) + H2O
Uses: Silica gel is used as a drying agent, as a catalyst and in chromatography.
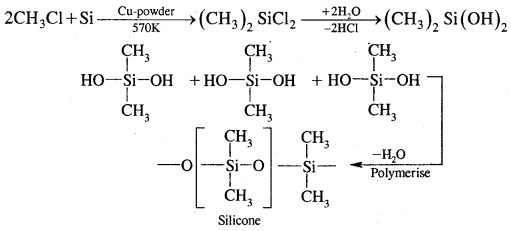
Silicones: They are a group of organosilicon polymers that have -R2SiO- as a repeating unit. They are prepared as follows:
industries for cracking of hydrocarbons and isomerisation, e.g., ZSM-5 (A type of zeolite) used to convert alcohols directly into gasoline. Hydrated zeolites are used as ion exchangers in softening hard water.