By going through these CBSE Class 11 Chemistry Notes Chapter 9 Hydrogen, students can recall all the concepts quickly.
Hydrogen Notes Class 11 Chemistry Chapter 9
→ Hydrogen: Hydrogen is the lightest atom with only one electron. Loss of this electron results in an elementary particle, the proton.
→ Isotopes of hydrogen: Protium (11H), Deuterium (D or 21H) & Tritium (T or 31H). Tritium is radioactive.
→ Water-Gas Shift Reaction: Dihydrogen is obtained on an industrial scale by this reaction.
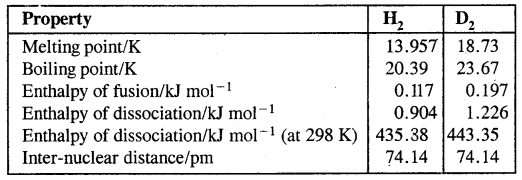
→ Bond dissociation Enthalpy: Bond dissociation enthalpy of dihydrogen is (435.88 KJ mol-1) is highest for a single bond between two atoms of any elements.
→ Hydrides: Dihydrogen combines with almost all the elements under appropriate conditions to form hydrides. Three types of hydrides as Ionic or saline, covalent or molecular hydrides & metallic or non-stoichiometric hydrides.
→ Hydrogen Economy: The basic principle of a hydrogen economy is the transportation & storage of energy in the form of liquid or gaseous dihydrogen. In fact, it has promising potential for use as a non-polluting fuel of the near future.
→ Water: It is of great chemical & biological significance. Water molecule is highly polar in nature due to its bent structure. This property leads to hydrogen bonding which is maximum in ice & least in water vapor. Its property to dissolve many salts, particularly in large quantity makes it hard & hazardous for industrial use.
Temporary & permanent hardness can be removed by the use of, zeolites & synthetic ion exchangers.
→ Heavy water: D2O is manufactured by the electrolysis of normal water. It is essentially used as a moderator in nuclear reactors.
→ Hydrogen peroxide: H2O2 has an interesting non-polar Structure & widely used as an industrial bleach & in pharmaceutical & pollution control treatment of industrial & domestic effluents.
→ Dihydrogen: Isotopes -Protium, Deuterium & Tritium, No. of neutrons-NIL, One, & Two Tritium is radioactive
→ Water-Gas: Mixture of CO & H2.
→ Synthesis Gas or Syn Gas: When water gas is used for the synthesis of methanol & a no. of hydrocarbons. It is also called synthesis gas or syngas.
→ Coal Gasification: The process of producing syngas from coal is called coal gasification.
→ Uses of dihydrogen: In ammonia synthesis & in nitrogenous fertilizers & for preparing Vanaspati Ghee, manufacturing of organic chemical, particularly methanol; HCl & hydrides. Used as rocket fuel in space research. It does not produce any pollution & releases greater energy per unit mass of fuel in comparison to Gasoline & other fuels.
→ Hydrides: Three types of hydrides:
- Ionic or Saline
- Covalent or molecular
- metallic or interstitial
→ Molecular hydrides:
- Electron deficient
- Electron precise
- Electron rich
→ Water: Colourless & tasteless liquid. The unusual properties of water in the condensed phase (liquid & solid states) are due to the presence of extensive hydrogen bonding between water molecules.
Str. of the water molecule
→ Hard & Soft Water: The presence of Magnesium & Calcium salts in the form of hydrogen bicarbonates chloride & sulfate in water make water hard. Hard water does not give lather with soap. Water-free from soluble salts of calcium & magnesium is called Soft Water. It gives lather with soap easily.
→ Removal of Hardness of Water: Temporary hardness by boiling of water & by dark’s method. Permanent hardness by treatment with washing soda, Calgon’s method, ion exchange method & synthetic resins method.
→ Hydrogen-peroxide: Hydrogen peroxide is an important chemical used in the pollution control treatment of domestic & industrial effluents.
→ Storage of H2O2: H2O2 decomposes slowly on exposure to light. In presence of metal surfaces or traces of alkali (present in glass- containers), the following reaction is catalyzed.
2H2O2 (l) → 2H2O(l) + O2(g)
It is, therefore, stored in wax-lined glass or plastic vessels in dark. Urea can be added as a stabilizer. It is kept away from dust because dust can induce explosive decomposition of the compound.
→ Uses of H2O2:
- As a hair bleach & a mild disinfectant. It is sold in the market as Perhydrol.
- In manufacturing chemicals that are used in high-quality detergents.
- In the synthesis of hydroquinone, tartaric acid & incertain food products & in pharmaceuticals (cephalosporin), etc.
- It is also used in environmentally green chemistry.
→ Heavy Water: D2O uses as a moderator in nuclear reactors & can be prepared by exhaustive electrolysis of water.
→ Hydrogen Economy: Hydrogen economy is an alternative. The basic principle of a hydrogen economy is the transportation & storage of energy in the form of liquid or gaseous dihydrogen. Nowadays, it is also used in fuel cells for the generation of electric power. Position of Hydrogen in the periodic table
Hydrogen is the first element in the periodic table. Its atom has only one proton and one electron. In the elemental form, it exists as H2 and is called dihydrogen. The electronic configuration of hydrogen is Is1. Alkali metals have an electronic configuration of ns1 which is similar. On the other hand like halogens (electronic configuration ns2np5, of 17th group) it is short by one electron than the corresponding noble gas configuration of He-1s2. Hydrogen thus resembles both alkali metals as well as halogens.
But hydrogen also differs from alkali metals and halogens in some other respects. Thus it is unique in its behavior and is, therefore, best placed separately in the periodic table.
Occurrence of Dihydrogen (H2):
It is the most abundant element in the universe (70% of the total mass of the universe) and is the principal element in the solar atmosphere. However, due to its light nature, it is much less abundant (0.15% by mass) in the earth’s atmosphere. In the combined form, it constitutes 15.4% of the earth’s crust and the oceans. In the combined form, besides in water, it occurs in plants, and animal tissues, carbohydrates, proteins, hydrides including hydrocarbons.
Isotopes of Hydrogen:
Hydrogen has three isotopes: protium 11H, deuterium, 21H or D, and tritium, 31H or T.
They differ from one another in the number of neutrons. Ordinary hydrogen, protium has no neutrons, deuterium has one and tritium has two neutrons in the nucleus.
Tritium is radioactive and is present as 1 atom per 1018 atoms of protium. 21H or D is also known as heavy hydrogen.
Table: Physical properties of Dihydrogen and Dideuterium
Preparation of Dihydrogen
Laboratory Preparation of Dihydrogen H2
(a) By the action of acids on metals: Metals (like Li. Na, Ba, Mg, Al, Zn, Fe, etc.) placed above hydrogen in the electrochemical series; when reacted with acids like HCl or dil. H2SO4 evolves hydrogen gas. Reaction with Li, K, Na, Ba, and Ca is violent while reaction with Zn, Fe, Al, and Mg is smooth.
Zn + H2SO4 → ZnSO4 + H2 (lab. method)
Fe + 2HCl → FeCl2 + H2
(b) By the action of alkalies on amphoteric metals Zn, Al, Pb, Sn, As, Sb, etc.)
Zn + 2NaOH → Na2ZnO2 (Sodium zincate ) + H2
2Al + 2NaOH + 2H2O → 2NaAlO2 (Sodium aluminate) + 3H2
Sn + 2KOH + H2O → K2SnO3 (Potassium stannate) + 2H2
(c) By the action of water on active metals (metals placed above electrochemical series)
1. Active metals like Na, K react at room temperature.
2Na + 2H2O (cold) → 2NaOH + H2 (violent)
Ca + 2H2O (cold) → Ca(OH)2 + H2 (smooth)
2. Less active metals like Zn, Mg, Al liberate hydrogen only on heating.
Mg + 2H2O (hot) → Mg(OH)2 + H2
3. Metals like Fe, Co, Ni, Sn can react only by passing steam.
3Fe (red hot) + 4H2O (steam) → Fe3O4 + 4H2
(d) By the action of water on a metal hydride:
LiH + H2O → LiOH + H2
CaH2 + 2H2O → Ca(OH)2 + 2H2
Commercial production of Dihydrogen: The commonly used processes are:
Electrolysis of acidified water, using platinum electrodes, is employed for the bulk preparation of dihydrogen.
![]()
(a) Hydrogen of high purity (> 99.95%) is obtained by electrolysis warm aqueous barium hydroxide between nickel electrodes.
(b) Reaction of steam on hydrocarbons or coke at high temperatures in the presence of catalyst yields hydrogen gas.
![]()
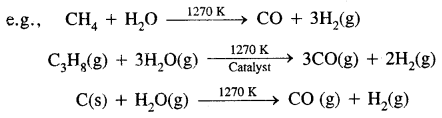
CO is converted to CO2 bypassing the gas’s steam over an iron oxide or cobalt oxide catalyst at 673K resulting in the generation of more H2.
This is called the water-gas shift reaction.
(c) Relatively smaller quantities of dihydrogen (1-17 m3 h-1) are obtained by passing a 1.1 molar mixture of vaporized methanol and water over a “base-metal chromite” type catalyst at 673 K. The mixture of hydrogen and carbon monoxide obtained is made to react with steam to give CO2 and more hydrogen.
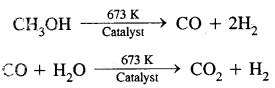
(d) It is also produced as a by-product of the brine electrolysis process for the manufacture of chlorine and sodium hydroxide. Presently-77% of the industrial hydrogen produced is from petrochemicals, 18% from coal, 4% from the electrolysis of aqueous solution, and 1 % from other sources.
Properties of Dihydrogen:
(a) Physical Properties:
- Hydrogen is colorless, odorless, and tasteless gas.
- It is the lightest element and also the lightest gas.
- It is sparingly soluble in water.
- Its critical temperature is very low (-236.9°C) at or below which can be liquefied by the application of suitable pressure. At -258.8°C it can be liquefied.
- Its molecule is diatomic, indicated by the ratio of its specific heats at constant pressure and constant volume (Cp/Cv = 1.40).
- It is adsorbed (occluded) by certain metals like Fe, Au, Pt, and Pd.
(b) Chemical properties:
1. Dihydrogen H2 combines with halogens (X2) to give hydrogen halides (HX). While the reaction with fluorine takes place even in the dark, with iodine a catalyst is required.
H2(g) + X2(g) → 2HX(g) (X = F, Cl, Br, I)
2. With dioxygen, dihydrogen forms water. The reaction is strongly exothermic
2H2(g) + O2(g) → 2H2O(1) ΔH° = -285.8 kJ mol-1
3. Reaction with dinitrogen it forms ammonia (NH3)
![]()
ΔH° = -92.6 kJ mol-1
4. Reaction with metals: With many metals, it combines at high temperatures to yield the corresponding hydrides.
H2(g) + 2M(g) → 2MH(s)
Where M is an alkali metal
5. Reaction with metal ions and metal oxides: It reduces
some metal ions in aqueous solution and oxides of metals (less active than iron) into corresponding metals.
H2(g) + Pd2+ (aq) → Pd(s) + 2H+(aq)
H2(g) + Cu2+ (aq) → Cu(s) + 2H+(aq)
in general: YH2(g) + MxOy(s) → xM(s) + yH2O(l)
Reaction with organic compounds: It reacts with many organic compounds in presence of catalysts to give useful hydrogenated products of commercial importance. For example;
- Hydrogenation of vegetable oils using nickel as catalyst gives edible fats (margarine and vanaspati ghee)
- Hydroformylation of olefins yields aldehydes which further undergo reduction to give alcohol.
H2 + CO + RCH = CH2 → RCH2CH2CHO
H2 + RCH2CH2CHO → RCH2CH2CH2OH
1. The largest single use of dihydrogen is in the synthesis of ammonia which is used in the manufacture of nitric acid and nitrogenous fertilizers.
2. Dihydrogen is used in the manufacture of vanaspati fat by the hydrogenation of polyunsaturated vegetable oils like soybean, cotton seeds, etc.
![]()
3. It is used in the manufacture of bulk organic chemicals, particularly methanol.
![]()
4. It is widely used for the manufacture of metal hydrides.
5. It is used for the preparation of hydrogen chloride, a highly useful chemical.
6. In metallurgical processes, it is used to reduce heavy metal oxides to metals.
7. Atomic hydrogen and oxy-hydrogen torches find a use for cutting and welding purposes. Atomic hydrogen atoms (produced by dissociation of dihydrogen with the help of an electric arc) are allowed to recombine on the surface to be welded to generate a temperature of 4000 K.
8. It is used as rocket fuel in space research.
9. Dihydrogen is used in fuel cells for generating electrical energy. It has many advantages over conventional fossil fuels and electric power. It does not produce any pollution and releases greater energy per unit mass of fuel in comparison to gasoline and other fuels.
Hydrides:
Dihydrogen under certain reaction conditions combines with almost all elements except noble gases to form binary compounds called hydrides expressed as EH [like MgH2] or EmHn (like B2H6).
They are of 3 types:
- Ionic or Saline or Salt-Like Hydrides
- Covalent or Molecular Hydrides
- Metallic or Non-Stoichiometric Hydrides
1. Ionic or Saline or Salt-Like Hydrides: Lighter metal hydrides like LiH, BeH, and MgH, have significant covalent character. Ionic hydrides like K+H. Na+H are crystalline, non-volatile, and non-conducting in solid-state. However, their melts conduct electricity and in electrolysis liberate dihydrogen gas at the anode which confirms the existence of H+ ions
![]()
They are generally formed by s-block elements which are highly electropositive in character. These hydrides are Stoichiometric. Saline hydrides react violently with water producing dihydrogen gas.
NaH(s) + H2O(aq) → NaOH(aq) + H2(g)
Lithium hydride is rather unreactive at a moderate temperature with O2 or Cl2. It is, therefore, used in the synthesis of other useful hydrides, e.g.
8 LiH + Al2Cl6 → 2LiAlH4 + 6 LiCl
2 LiH + B2H6 → 2LiBH4
2. Molecular hydrides/[Covalent Hydrides]: These are formed by elements of highly electronegative elements (viz non-metals) which share electron(s) with hydrogen. In most cases, bonds are covalent in character, although in some cases (eg HF) bond is partly ionic in character. These have molecular lattices. The molecules are held together by weak van der Waal’s forces. These hydrides are soft, have low m.p. and b.p. They have low electrical conductivity.
The stability decreases progressively down a group, e.g.
NH3 > PH3 > AsH3 > SbH3 > BiH3
In a period the stability increases with increasing electronegativity of the element forming the hydride.
e.g., CH4 < NH3 < H2O < HF
These become increasingly acidic in character on moving from left to right along a given row in the periodic table. Thus, while NH3 is a weak base, H2O is neutral and HF is acidic. Similarly, in the next row, while PH3 is a weak base, H2S is a weak acid and HCl is highly acidic.
These are used as reducing agents.
Molecular hydrides are further classified according to the relative numbers of electrons and bonds in their Lewis structure into:
- Electron-deficient,
- Electron-precise, and
- Electron-rich hydrides.
An electron-deficient hydride, as the name suggests, has too few electrons for writing its conventional Lewis structure. Diborane (B2H6) is an example. In fact, all elements of group 13 will form electron-deficient compounds. They act as Lewis acids i.e., electron acceptors.
Electron-precise compounds have the required number of electrons to write their conventional Lewis structures. All elements of group 14 form such compounds (e.g., CH4) which are tetrahedral in geometry.
Electron-rich hydrides have excess electrons which are present as lone pairs. Elements of groups 15-17 form such compounds. (NH3 has 1-one pair, H2O-2, and HF-3 lone pairs). They will behave as Lewis bases i.e., electron donors. The presence of lone pairs on highly electronegative atoms like N, O, and F in hydrides results in hydrogen bond formation between the molecules. This leads to the association of molecules.
3. Metallic or non-stoichiometric (or Interstitial) Hydrides:
These are formed by many (d-block or f-block elements. However, metals of groups 7, 8, and 9 do not form hydrides. These hydrides conduct heat and electricity. They are non-stoichiometric. Hydrogen atoms occupy interstitial places in the lattices of metals. They are reducing’ agents and give out hydrogen easily. Hydrogen in them is present in atomic form.
Water:
A major part of all living organisms is made up of water. The human body has about 85 % and some plants have as much as 95 % water. It is a crucial compound for the survival of all life forms.
Physical properties of water:
It is a tasteless and colorless liquid.
The molecular mass of H2O is = 18.0151 g mol-1
Melting point = 273.0 K
Boiling point = 373.0 K
enthalpy of formation = – 285.9 kJ mol-1
Enthalpy of fusion = 6.01 kJ mol+
Enthalpy of vaporisation (373 K) = 40.66 kJ mol-1
Density (at 298 K) = 1.00 g cm–3.
The unusual properties of water in the condensed phase (liquid and solid states) are due to the presence of extensive hydrogen bonding between water molecules. It boils at a higher temperature than H2S or H2Se only because of hydrogen bonding.
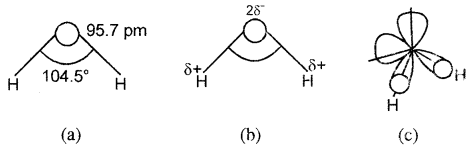
Structure of Water:
In the gas phase, water is a bent molecule with a bond angle of 104.5° and an O-H bond length of 95.7 pm as shown in Fig (a). It is a highly polar molecule, (Fig.(b)). Its orbital overlap picture is shown in Fig. (c) In the liquid phase water molecules are associated together by hydrogen bonds.
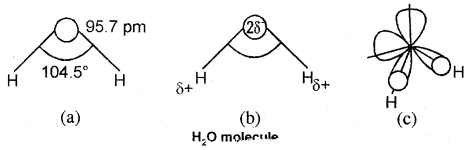
(a) The bent structure of water;
(b) the water molecule as a dipole and
(C) the orbital overlap picture In water molecule.
Structure of Ice:
Ice has a highly ordered three-dimensional hydrogen-bonded structure as shown in Fig. Examination of ice crystals with x-rays shows that each oxygen atom is surrounded tetrahedrally by four other oxygen atoms at a distance of 276 pm.
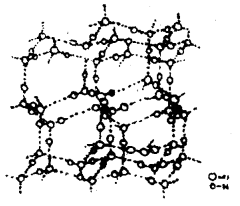
The structure of Ice
Hydrogen bonding gives the ice a rather open type structure with wide holes. These holes can hold some other molecules of appropriate size interstitially.
Chemical Properties Of Water:
1. Amphoteric Nature: It has the ability to act as an acid as well as a base, i.e., it behaves as an amphoteric substance. In the Bronsted sense, it acts as an acid with NH3 and a base with H2S.
H2O(l) + NH3(aq) ⇌ NH+4 (aq) + OH–(aq)
H2O(l) + H2S(aq) ⇌ H3O+ (aq) + HS– (aq)
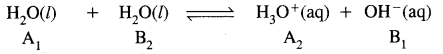
The auto-protolysis (self-ionization) of water takes place as follows:
2. Reduction Reaction:
2H2O(l) + 2Na(s) → 2NaOH(aq) + H2(g)
3. Oxidation Reaction:
2F2(g) + 2H2O(aq) → 4H+(aq) + 4F–(aq) + O2
4. Hydrolysis Reaction:
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
SiCl4(l) + 2H2O(l) → SiO2(s) + 4HCl(aq)
N3-(s) + 3H2O(l) → NH3(g) + 3OH–(aq)
5. Hydrates Formation: From aqueous solutions, many salts can be crystallized as hydrated salts.
- Coordinated water e.g. [Cr(H2O)6]3+ 3Cl–
- Interstitial water e.g. BaCl2.2H2O.
- Hydrogen bonded water e.g., [Cu(H2O)4]2+ SO4 H2O in CuSO4. 5H2O.
Hard and Soft Water:
The presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride, and sulfate in water makes water Hard. Hard Water does not give lather with soap. Water-free from soluble salts of calcium and magnesium is called Soft Water. It gives lather with soap easily.
Hard water forms scum/precipitate with soap.
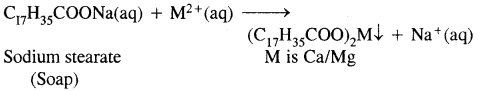
Disadvantages of using hard water
- It is unsuitable for laundry.
- It is harmful to boilers due to the deposition of salts as a scale on the walls of boilers. This reduces the efficiency of boilers.
There are two types of hardness:
(A) Temporary Hardness: It is due to the presence of the bicarbonates of calcium and magnesium, viz., Ca(HCO3)2 and Mg(HCO3)2
Temporary hardness can be removed by
1. Boiling
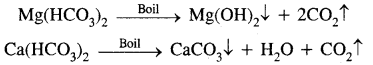
These precipitates are removed by filtration. The filtrate obtained is soft water.
2. Clark’s method: Lime water is added to hard water in Clark’s process to remove the precipitates of CaCO3 formed.
Ca(HCO3)2 + Ca(OH)2 → 2CaCO3↓ + 2H2O
Mg(HCO3)2 + 2Ca(OH)2 → 2CaCO3↓ + Mg(OH)2↓ + 2H2O
(B) Permanent Hardness: It is due to the presence of soluble salts of Mg and Ca in the form of chlorides and sulfates:
MgCl2, CaCl2, MgSO4, CaSO4. It can be removed by the following methods:
1. Treatment with washing soda (Na2CO3)
MCl2 + Na2CO3 → 4 MCO3↓ + 2NaCl
MSO4 + Na2CO3 → MCO3↓ + Na2SO4
M = Mg, Ca.
2. Calgon’s Method: Sodium hexametaphosphate Na6P60lg is commercially called Calgon. When added to hard water, the following reactions take place.
Na6P6O18 → 2Na+ + Na4P6O182-[M = Mg, Ca]
M2+ + Na4P6 O182- → [Na2MP6O18]2-
The complex anion is not harmful.
3. Permutit Method: Permutit is an artificial zeolite- chemically it is sodium orthosilicate (Na2Al2Si2O8. xH2O). Permutit removes cations like Ca2+, Mg2+, and Fe2+ and releases an equivalent number of Na+ ions. For simplicity, it can be written as Na Z. When added to water the following reaction takes place
2NaZ + M2+(aq) → MZ2(s) + 2Na+(aq); M = Mg, Ca
Permutit/zeolite is said to be exhausted when all the sodium in it is used up. It is regenerated when treating it with an aqueous sodium chloride solution.
MZ2(S) + 2NaCl(aq) → 2NaZ + MCl2(aq)
4. By the use of ion exchange resins (synthetic resins): This method removes all cations and anions present in water by means of ion-exchange resins. Water is first passed through cation exchange resins (giant organic molecules with-SO3H or —COOH groups), which remove the cations like Na+, Ca2+, Mg2+ and others by exchange with H+. The resulting water is now passed through anion exchange resins (giant organic molecules with — NH2 group) which remove the anions like Cl–, SO4– and NO3 by exchange with OH–.
Hydrogen Peroxide (H2O2):
It is an important chemical used in the pollution control treatment of domestic and industrial effluents.
Preparation:
1. By the reaction of sulphuric acid or phosphoric acid on hydrated barium peroxide (BaO2).
(a) BaO2.8H2O + H2SO4 → BaSO4(g) + H2O2 + 8H2O
Anhydrous barium peroxide does not react readily with sulphuric
acid because a coating of insoluble barium sulfate is formed on its surface which stops further action of the acid. Hence hydrated barium peroxide, BaO2,.8H2O must be used.
(b) 3BaO2 + 2H3PO4 → Ba3(PO4)2 + 3H2O2
Ba3(PO4)2 + 3H2SO4 → 2BaSO4(s) + 2H3PO4
Treatment with phosphoric acid is preferred to H2SO4 because soluble impurities like barium persulphate (from BaO2.8H2O+ H2SO4) tend to decompose H2O2 while H3PO4 acts as a preservative (negative catalyst for H2O2). Moreover, excess barium peroxide should be avoided as it tends to decompose H2O2.
BaO2 + H2O2 → BaO + H2O + O2
In both cases, BaSO4 is removed by filtration and hence more or less a fuse H2O2 solution is obtained by this method.
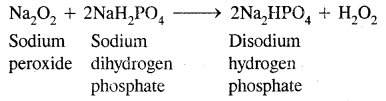
1. By adding the calculated quantity of sodium peroxide to a 20 % ice-cold sulphuric acid solution (Merck’s process):
Na2O2 + H2SO4 → Na2SO4 + H2O2
Sodium sulfate is removed by cooling when crystals of Na2SO4 10H2O separate out.
In this method, sulphuric acid can be replaced by NaH2PO4
Manufacture of Hydrogen peroxide:
1. By electrolysis of 50 % sulphuric acid to give Perdisulphuric acid (H2S2O8) which on distillation yields 30% solution of hydrogen peroxide.
2H2SO4 → 2H+ + 2HSO4–
At cathode (Cu coil):
2H+ + 2e– → 2H + H2 ↑
At anode (Pt)
2HSO4– → 2HSO4 + 2e–
2HSO4 → H2S2O8 (Persulphuric acid)
H2S2O8 + 2H2O → 2H2SO4 + H2O2
Alternatively, electrolysis may be done with ammonium hydrogen sulfate (ammonium sulfate + H2SO4).
(NH4)2SO4 + H2SO4 → 2NH4HSO4
NH4HSO4 → H+ + NH4SO4
At cathode 2H+ + 2e– → H2
At anode 2NH4SO4 → (NH4)2S2O8 + 2e–
The ammonium persulphate formed is removed and quickly distilled with dil. H2SO4 under reduced pressure to give hydrogen peroxide.
![]()
2. By the auto-oxidation of 2-ethyl anthraquinone: In this process, the air is passed through a 10% solution of 2-ethyl anthraquinone in a mixture of benzene and higher alcohol.
![]()
The resulting 2-ethyl anthraquinone is then reduced by hydrogen in presence of palladium as a catalyst. Thus the continuity of the process is maintained and the process needs only H2, atmosphere 02, and water as the major raw materials.
Physical Properties of hydrogen peroxide:
- Pure hydrogen peroxide is a pale blue syrupy liquid.
- It is an unstable liquid and decomposes into water and oxygen either on standing or on heating.
- Hydrogen peroxide is diamagnetic.
- In the pure state, its dielectric constant is 93.7 which increases with dilution.
- It is more highly associated with hydrogen bonding than water.
- Pure hydrogen peroxide is weakly acidic in nature while its aqueous solution is neutral.
Chemical properties:
It acts as an oxidizing as well as a reducing agent in both acidic and alkaline media. Simple reactions are described below.
1. Oxidising action in acidic medium
2Fe2+(aq) + 2H+(aq) + H2O2(aq) → 2Fe3+(ag) + 2H2O(l)
PbS(s) + 4H2O2(aq) → PbSO4(s) + 4H2O(l)
2. Reducing action in acidic medium
2MnO4 + 6H+ + 5H2O2 → 2Mn2+ + 8H2O + 5O2
HOCl + H2O2 → H3O+ + Cl– + O2
3. Oxidising action in basic medium
2Fe2+ + H2O2 → 2Fe3+ + 2OH–
Mn2+ + H2O2 → Mn4+ + 2OH–
4. Reducing action in basic medium
I2 + H2O2 + 2OH– → 2I– + 2H2O + O2
2MnO4– + 3H2O2 → 2MnO2 + 3O2 + 2H2O + 2OH–
Storage of H2O2
H2O2 decomposes slowly on exposure to light.
2H2O2(l) → 2H2O(l) + O2(g)
In the presence of metal surfaces or traces of alkali (present in glass containers), the above reaction is catalyzed. It is, therefore, stored in wax-lined glass or plastic vessels in dark. Urea can be added as a stabilizer. It is kept away from dust because dust can induce explosive decomposition of a compound.
Structure of H2O2
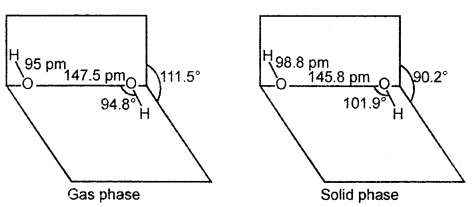
Hydrogen peroxide molecule has a non-polar structure. The molecular dimensions in the gas phase and chemical phase are shown in Fig.
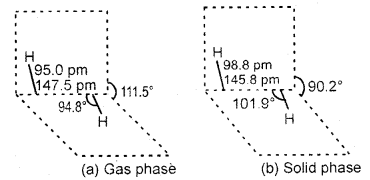
(a) H2O2 structure (gas phase) Dihedral angle 111.5° (b) H,0, (so, id phase at 110 K. The dihedral angle is reduced to 90.2°.
Uses Of Hydrogen Peroxide:
- In daily life, it is used as hair bleach and as a mild disinfectant. As an antiseptic, it is sold in the market as per hydro.
- It is used to manufacture chemicals like sodium perborate and per-carbonate, which are used in high-quality detergents.
- It is used in the synthesis of hydroquinone, tartaric acid, and certain food products and Pharmaceuticals (cephalosporin), etc.
- It is employed in the industries as a bleaching agent for textiles, paper pulp, leather, oils, fats, etc.
- Nowadays it is also used in Environmental (Green) Chemistry. For example, in pollution control treatment of domestic and industrial effluents, oxidation of cyanides, restoration of aerobic conditions to sewage wastes.
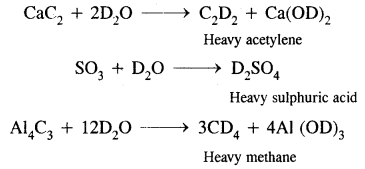
Heavy Water (D2O):
It is extensively used as a moderator in nuclear reactors and in exchange reactions for the study of reaction mechanisms. It was first prepared by Urey by the exhaustive electrolysis of water.
It is used for the preparation of other deuterium compounds. For example,
Volume Strength Of Hydrogen Peroxide:
H2O2 is miscible with water in all proportions and forms a hydrate H2O2.H2O (mp 221 K). A 30% solution of H2O2 is marketed as “100 Volume” hydrogen peroxide. It means that one milliliter of 30% H2O2 solution will give 100V of oxygen at STP. Commercially, it is marketed as 10V. It means it contains 3% H2O2.
Problem:
Calculate the strength of a 10 volume solution of hydrogen peroxide.
Answer:
10 volume solution of H2O2 means that 1L of this H2O2 will give 10L of oxygen at STP
2H2O2 (l) → O2(g) + H2O(l)
2 × 34 = 68g 22.4 L at STP
22.4 L of 02 at STP is produced from H2O2 = 68g
10 L of O2 at STP is produced from H2O2 = \(\frac{68 \times 10}{22.4}\)g
= 30.36g
Therefore, the strength of H2O2 in 10 volume H2O2 = 30.36g L-1.
Dihydrogen as a Fuel:
It releases large quantities of heat on combustion. On mass for mass basis H2(g) can release, more energy than petrol (about three times). Moreover, pollutants in the combustion of dihydrogen will be less than petrol. The only pollutant will be oxides of dinitrogen (due to the presence of dinitrogen as an impurity with dihydrogen).
This, of course, can be minimized by injecting a small amount of water into the cylinder to lower the temperature so that reaction between dinitrogen and dioxygen may not take place.
However, the mass of the containers in which dihydrogen will be kept must be taken into consideration, A cylinder of compressed dihydrogen weighs about 30 times as much as a tank of petrol containing the same amount of energy. Also, dihydrogen gas is converted into a liquid state by cooling to 20K.
This would require expensive insulated tanks. Tanks of metal alloy like NaNi5, Ti-TiH2, Mg-MgH2, etc. are in use of storage of dihydrogen in small quantities. These limitations have prompted researchers to search for alternative techniques to use dihydrogen in an efficient way.
In this view Hydrogen Economy is an alternative. The basic principle of a hydrogen economy is the transportation and storage of energy in the form of liquid or gaseous dihydrogen. The advantage of a hydrogen economy is that energy is transmitted in the form of dihydrogen and not as electric power.
It is for the first time in the history of India that a pilot project using dihydrogen as fuel was launched in Oct 2005 for running automobiles. Initially, 5 % dihydrogen has been mixed in CNG for use in four-wheeler vehicles. The percentage of dihydrogen would be gradually increased to reach the optimum level. Nowadays, it is also used in fuel cells for the generation of electric power.