Here we are providing Class 11 chemistry Important Extra Questions and Answers Chapter 12 Organic Chemistry: Some Basic Principles and Techniques. Chemistry Class 11 Important Questions are the best resource for students which helps in Class 11 board exams.
Class 11 Chemistry Chapter 12 Important Extra Questions Organic Chemistry: Some Basic Principles and Techniques
Organic Chemistry: Some Basic Principles and Techniques Important Extra Questions Very Short Answer Type
Question 1.
What type, of hybridisation, is involved in
(i) planar and
Answer:
sp2
(ii) linear molecules?
Answer:
sp.
Question 2.
Arrange the following in increasing order of C – C bond strength:
C2H6, C2H4C2H2.
Answer:
C2H6 < C2H4 < C2H2.
Question 3.
Arrange the following in decreasing order of C — C bond length:
Answer:
C2H6 > C2H4 > C2H2.
Question 4.
What is the type of hybridisation of C atoms in benzene?
Answer:
It is an sp2 type of hybridisation.
Question 5.
What are isomers?
Answer:
Compounds having the same molecular formula but different physical and chemical properties are called isomers.
Question 6.
Select electrophiles out of the following:
H+ Na+, Cl–, C2HSOH, AlCl3, SO3, CN–, CH3CH2+,: CCl2, R-X.
Answer:
H+, Na+, A1Cl3, SO3, CH3CH2+,: CCl2, R-X.
Question 7.
Select nucleophiles from the following.
BF3 NH3 OH–, R-X, C2H5OH, H3O+, NO2, CN–.
Answer:
NH3, OH, C2H5OH, CN
Question 8.
Give the I.U.P.A.C. names of the following compounds
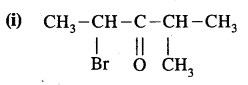
Answer:
2-Bromo-4 – methyl pent-3- one
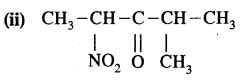
Answer:
4-Methyl-2-nitro pent – 3 – one
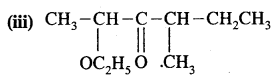
Answer:
2 – Ethoxy – 4 – methoxypent – 3 – one
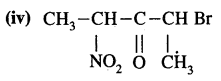
Answer:
2-Bromo-4-nitro pent-3-one
(v) (CH3)4C
Answer:
2, 2-Dimethylpropane
(vi) (CH3)2CHCOOH.
Answer:
2-Methyl propanoic acid.
Question 9.
What is a functional group?
Answer:
The atom or group of atoms present in a molecule that determines its chemical properties is called the functional group.
Question 10.
Arrange the following in increasing order of-I effect.
(i) -NO2, -COOH, -F, -CN, – I.
Answer:
-I < -F < -COOH < -CN <NO2.
Question 11.
Arrange the following in decreasing order of + I effect: CH3-, D, (CH3)3C-, (CH3)2CH-, CH3-CH22–
Answer:
(CH3)3C → (CH3)2CH → CH3-CH2 → CH3 → D
Question 12.
Name the alkyl groups derived from isobutane.
Answer:
(CH3)2CH – CH2– isobutyl and (CH3)3C – tertiary butyl.
Question 13.
What type of isomerism is shown by butane and isobutane.
Answer:
Chain or nuclear isomerism.
Question 14.
Write the tautomer of acetaldehyde and its I.U.P.A.C. name.
Answer:
CH2 = CH-OH and its I.U.P.A.C. name is Eth-1-en-1-ol,
Question 15.
Give one example of functional isomerism.
Answer:
CH3– CH2– OH and CH3 – O – CH3.
Question 16.
Give one example of position isomerism.
Answer:
CH3 – CH2 – CH2OH and CH3CH(OH) CH3 .
Question 17.
Draw the structure of the tautomer of phenol and write its I.U.P.A.C name.
Answer:
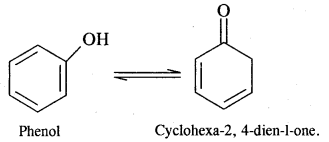
Question 18.
A compound is formed by the substitution of two chlorine atoms for two hydrogen atoms in propane. What is the number of structural isomers possible?
Answer:
Four
1. CH3CH2CHCl2
2. CH3CHClCH2Cl
3. CH3CCl2CH3
4. ClCH2 – CH2 – CH2Cl
Question 19.
Write the metamer of diethyl ether. What is its I.U.P.A.C. name?
Answer:
- CH3O CH3 CH2 CH3 Its I.U.P.A.C. name is 1 -Methoxypropane.
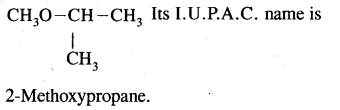
Question 20.
Give the I.U.P.A.C. name of CH2 = CH-CH (CH3)2
Answer:
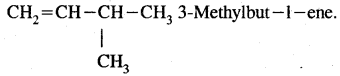
Question 21.
How many cr and it bonds are present in each of the following molecules?
(a) H-C = CCH=CH-CH3
Answer:
No. of σC-C = 4;
No. of σC-H = 6.
Total no. of σ bonds =10.
Total no. of πC=C bonds = 2 + 1=3.
(b) CH2=C=CH-CH3.
Answer:
(b) No. of σC-C = 3 bonds = 3,
No. of σC-H = 6;
Total No. of , σ-bonds = 3 + 6 = 9
No. of πC=C bonds = 2.
Question 22.
What is the shape of the following molecules
(a) H3 C = O
Answer:
HCHO formaldehyde C: sp2 hybridised; shape: Trigonal planar
(b) CH3F
Answer:
CH3F; C = sp3 hybridised shape: Tetrahedral
(c) HON.
Answer:
H-C ≡ N; C is sp hybridised, HCN is linear.
Question 23.
Write the T.U.P.A.C. name of
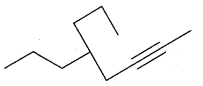
Answer:
Compound is
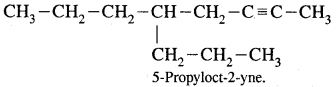
Question 24.
Give the condensed and bond-line structural formula for
(a) Penta-1, 4-dien
Answer:
CH2 = CH – CH2 – CH = CH2
![]()
(b) Hexa-1, 3, 5 triene.
Answer:
CH2 = CH – CH = CH – CH = CH
Its bond line structure is
![]()
Question 25.
Write the I.U.P.A.C. names of HOOC-C ≡ C-COOH and
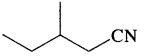
Answer:
But-2-yne – 1,4-dioic acid and 3-Methyl pentanenitrile.
Question 26.
How will you purify essential oils?
Answer:
Essential oils are volatile and are insoluble in water, Therefore, they are purified by steam distillation.
Question 27.
A liquid (1.0 g) has three components. Which technique will you employ to separate them?
Answer:
Column chromatography.
Question 28.
A reaction carried out using aniline as a reactant as well as a solvent. How will you remove unreacted aniline?
Answer:
By steam distillation.
Question 29.
How will you separate a mixture of O-nitrophenol and /Mutrophenol?
Answer:
Steam distillation O-Nitrophenol being volatile distils over along with water while p-nitrophenol being non-volatile remains in the flask.
Question 30.
How will you purify a liquid having non-volatile impurities?
Answer:
Simple distillation will give pure liquid while the non-volatile impurities in the flask as residue.
Question 31.
Suggest a method to purify
(i) Kerosene containing water
Answer:
By solvent extraction using a separating funnel.
(ii) a liquid that decomposes at its boiling point.
Answer:
Distillation under reduced pressure.
Question 32.
Suggest methods for the separation of the following mixtures:
(i) A mixture of liquid A,(B. Pt. =365 K) and liquid B (b.p.356 K)
Answer:
Fractional distillation B.Pts of two liquids differ only by just 9°.
(ii) A mixture of liquid C (b.p. 353 K) and liquid D (b.p. 413 K).
Answer:
Simple distillation, since B.Pts of two liquids, are wide apart.
Question 33.
Name two compounds that do not contain halogen but give a positive Beilstein test.
Answer:
Urea and thiourea give a positive Beilstein test due to the formation of volatile cupric cyanide.
Question 34.
Lassaigne’s test is not shown by diazonium salts. Why?
Answer:
Diazonium salts lose N2 on heating much before they have a chance to react with fused sodium metal.
Question 35.
What type of compounds are purified by sublimation?
Answer:
Substances whose vapour pressures become equal to atmospheric pressure much below their melting point.
Question 36.
How will you separate iodine from sodium chloride?
Answer:
By sublimation.
Question 37.
Name two methods that can be safely used to purify aniline.
Answer:
Vacuum distillation and steam distillation.
Question 38.
Define the term elution as applied to column chromatography.
Answer:
The process of extraction of different adsorbed compounds from the column by means of a suitable solvent is called elution.
Question 39.
Suggest a suitable technique of separating naphthalene from kerosene oil present in a mixture.
Answer:
Simple distillation.
Question 40.
What conclusion would you draw if during Lassaigne’s test a blood-red colouration is obtained?
Answer:
It shows the presence of N & S together in the compound.
Question 41.
What type of organic compounds cannot be Kjeldahlised?
Answer:
A compound containing an N atom in the ring or the presence of – NO2, (nitro) and — N =N -(azo) in them.
Question 42.
Can we estimate oxygen in the organic compound?
Answer:
No. it is estimated indirectly by subtracting the percentage of all elements present in an organic compound from 100.
Question 43.
Why do we use copper spiral in Duma’s method?
Answer:
To reduce back any oxides of nitrogen formed during combustion to nitrogen.
Question 44.
Give two examples of adsorbents used in chromatography.
Answer:
- Alumina gel (Al2O3) and
- Silica gel SiO2.
Question 45.
Is Lassaigne’s extract neutral, acidic or alkaline?
Answer:
It is alkaline.
Question 46.
The empirical formula of a compound is CH2. It’s one mole has a mass of 42 g. What is its molecular formula?
Answer:
Molecular formula = n × empirical formula
= \(\frac{42}{14}\) × CH2 = C3H6
Question 47.
In which C-C bond of CH3CH2CH2Br, the inductive effect is expected to be the least?
Answer:
The magnitude of the inductive effect diminishes as the number of intervening bonds increases. The effect is least in the C3-H bond.
Question 48.
Can you use K in place of Na for fusing an organic compound in Lassaigne’s test?
Answer:
No, because K is more reactive than Na.
Question 49.
Which solution is used to absorb CO2 produced during combustion?
Answer:
KOH solution is used to absorb CO2 gas.
Question 50.
What is the cause of geometrical isomerism in alkenes?
Answer:
Alkenes have a π-bond & the restricted rotation around the π-bond gives rise to geometrical isomerism.
Organic Chemistry: Some Basic Principles and Techniques Important Extra Questions Short Answer Type
Question 1.
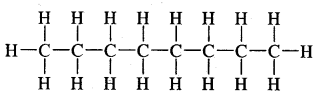
Expand each of the following bond-line formulae to show all the atoms including carbon and hydrogen.
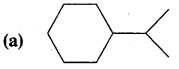
Answer:
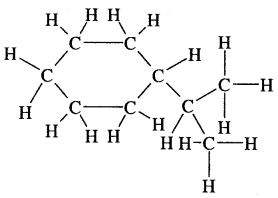
Answer:
Answer:
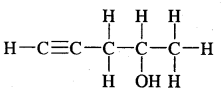
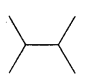
Answer:
Question 2.
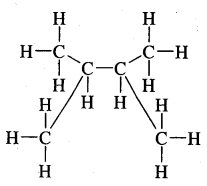
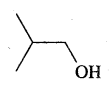
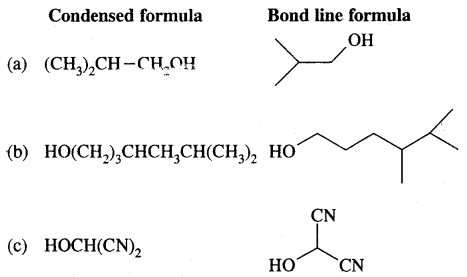
For each of the following compounds, write a more condensed and also their bond line formulae.
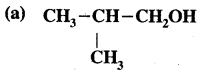
Answer:
Condensed formulae are
(CH3)2CH CH2 OH
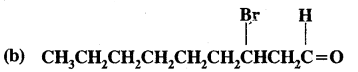
Answer:
CH3(CH2)5 CHBr CH2 CHO
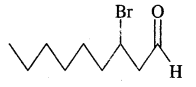
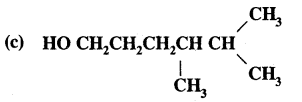
Answer:
HO(CH2)3 CH(CH3) CH(CH3)2
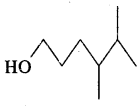
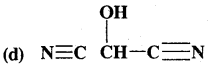
Answer:
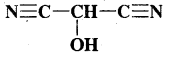
HOCH(CN)2
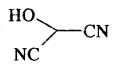
Question 3.
What is the type of hybridisation of each carbon in the following compounds?
(a) CH3Cl
Answer:
![]()
(b) (CH3)2CO
Answer:
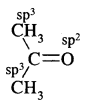
(c) CH3CN
Answer:
![]()
(d) HCONH2
Answer:
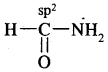
(e) CH3CH = CHCN.
Answer:
![]()
Question 4.
What is the shape of the following molecules:
(a) H2C = O
Answer:
In H2C = O; C is sp2 hybridised, hence its shape is H trigonal planar
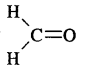
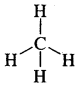
(b) CH3F
Answer:
In CH3 -F; C is sp3 hybridized
∴ it is tetrahedral
(c) H-C ≡ N?
Answer:
In H-C ≡ N; C is sp-hybridized, hence HCN is linear
H—C ≡ N.
Question 5.
Give the I.U.P. A.C. names of the following compounds:
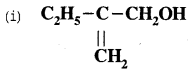
Answer:
2-Ethylprop-2-en-l-ol
(ii) CH3 – CH = CH COOH
Answer:
But-2-en-l-oic acid
(iii) (CH3)2C = CHCOCH3
Answer:
4-Methylpent-3-en-2-one
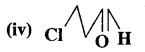
Answer:
3-Chloropropanal
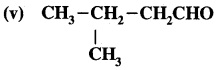
Answer:
3-Methylbutane-l-al
(vi) CH2 = CH – CN.
Answer:
Prop-2-en-1-nitrile.
Question 6.
Write the I.U.P.A.C. names of
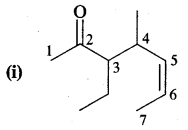
Answer:
3-Ethyl-4-methylhept-5-en-2-one
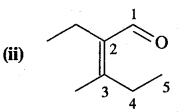
Answer:
2-Ethyl-3-methylpent-2-en-1 -one.
Question 7.
Write 1.U.P.A.C. names of
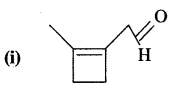
Answer:
2-[2-methylclo but-l-enyl] ethanal
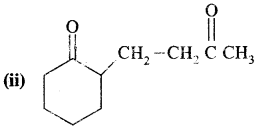
Answer:
2-(3-Oxobutyl) cyclohexane-1 one
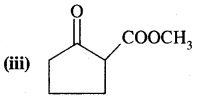
Answer:
Methyl (2-oxo cyclopentane-1-carboxylate
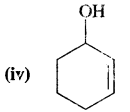
Answer:
Cyclohex-2-en-l-ol
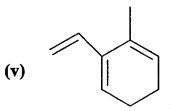
Answer:
2-Ethenyl-3-methyl-cyclohexa-l, 3-diene
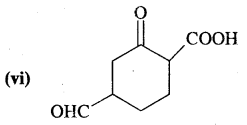
Answer:
4-Formyl-2-oxo-cyclohexane-l carboxylic acid.
Question 8.
Draw the structures of
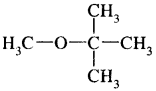
(i) Methyl t-butyl ether
Answer:
(ii) 2-Chloro-1, 1, 1-trifluoro ethane
Answer:
F3C – CH2Cl
(iii) 2-Methyl buta, 1, 3-diene
Answer:
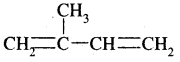
(iv) Pent-2-en-l-ol
Answer:
CH3 – CH2 – CH = CH – CH2OH
(v) Cyclo hex-2-en-l-ol
Answer:
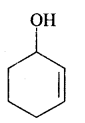
(vi) l-Bromo-3-chloro cyclohex-1-ene
Answer:
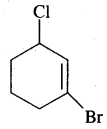
Question 9.
Write I.U.P.A.C. names of
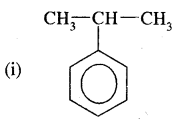
Answer:
(2-Isopropyl) benzene
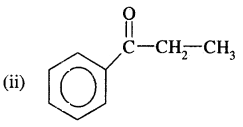
Answer:
1-Phenylpropane-l-one
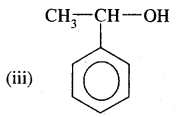
Answer:
1 -Phenylethan-1 -ol
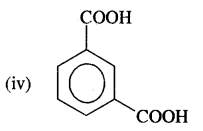
Answer:
1, 3-Benzene dicarboxylic acid
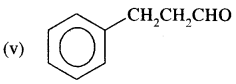
Answer:
3-Phenylpropanal
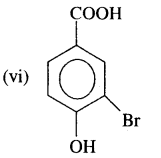
Answer:
3-Boromo-4-hydroxybenzoic acid
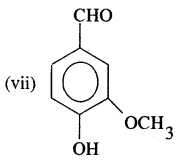
Answer:
4-Hydroxy-3-methoxy benzaldehyde.
Question 10.
Write the structure of
(i) O-Ethyl anisole
Answer:
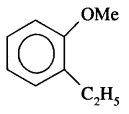
(ii) p— nitroaniline
Answer:
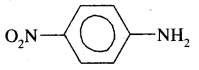
(iii) 4-Ethyl-I-fluoro-2-nitrobenzene.
Answer:
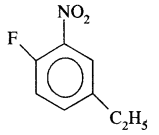
Question 11.
Which is more polar bond in the following pairs of molecules
(a) H3C-H, H3C-Br
Answer:
C —Br since Br is more electronegative than H.
(b) H3C-NH2, H3C-OH
Answer:
C—O since O is more electronegative than N.
(c) H3C-OH, H3C-SH.
Answer:
C — O since O is more electronegative than S.
Question 12.
In which C-C bond of CH3CH2CH2Br, the inductive effect is expected to be the least.
Answer:
The magnitude of the inductive effect decreases with distance from the active centre and hence this effect is least in C2—C3 bond.
![]()
Question 13.
Write the resonance structures of
(a) CH3COO- and
Answer:
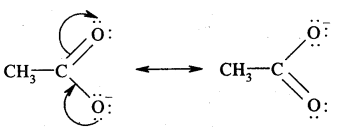
(b) CH6H5NH2. Show the movement of electrons by curved arrows.
Answer:
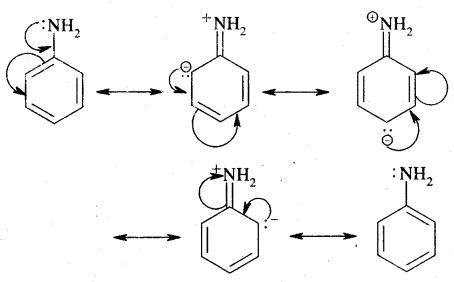
Question 14.
Which of the following pairs of structures do not constitute resonance structures?
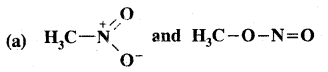
Answer:
They differ in the position of atoms and hence are not resonance structures.
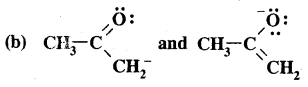
Answer:
They are a pair of resonance structures as they differ in the position of electrons.
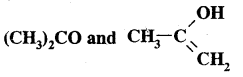
Answer:
They differ in the position of atoms and so are not resonance structures. They are tautomers.
(d) CH3CH = CHCH3 and CH3CH2CH=CH2
Answer:
They are not resonance structures as they differ in the position of atoms.
Question 15.
Write the resonance structures of CH2 = CH-CHO and arrange them in decreasing order of stability.
Answer:
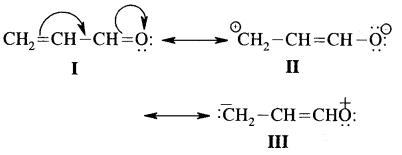
The structure I is most stable as each C & O have octets completed and there is no charge on either of them.
II & III involve charge separation hence both are less stable than I. However II is more stable than III because in II more electronegative oxygen carries a negative charge.
Thus decreasing order of stability is I > II > III.
Question 16.
Using curved arrow notation show the formation, of reactive intermediates when the following covalent bonds undergo heterolytic fission.
(a) CH3-SH3
Answer:
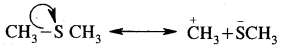
(b) CH3-CN
Answer:
![]()
(c) CH3-CU
Answer:
![]()
Question 17.
Giving proper justification categorise the following molecules/ ions as nucleophiles or electrophiles:
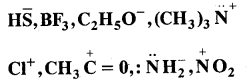
Answer:
![]()
are all nucleophiles as each one of them has one or more lone pairs of electrons to donate.
![]()
are all electrophiles. All these species have a sextet of electrons around positive centres.
Question 18.
A mixture contains two components A and B. The solubilities of A and B in the water near its boiling point are 10 grams per 100 ml and 2g per 100 ml respectively. How will you separate A and B from this mixture?
Answer:
Fractional crystallisation. When the saturated hot solution of this mixture is allowed to cool, the less soluble component B crystallises out first leaving the more soluble component B in the mother liquor.
Question 19.
A mixture containing benzoic acid and nitrobenzene is given to you. Using an appropriate chemical reagent, how will you proceed to separate them?
Answer:
The mixture is shaken with a dilute solution of NaHCO3 and extracted with-ether or chloroform when nitrobenzene goes into the organic layer; Distillation of this will yield nitrobenzene. The aqueous layer is acidified with dil. HCl and the solution are cooled.
Filteration gives benzoic acid.
C6H5COOH + NaHCO3 → C6H5COONa + CO2 + H2O.
C6H5COONa + HCl (dil.) → C6H5COOH + NaCl.
Question 20.
The Rf value of A and B in a mixture determined by TLC in a solvent mixture are 0.65 and 0.42 respectively. If the mixture separated by column chromatography using the same solvent mixture as a mobile phase, which of the two components A or B will elute first? Explain.
Answer:
Since the Rf value of A is 0.65, therefore, it is less strongly adsorbed as compared to component B with an Rf value of 0.42. Therefore an extraction in column chromatography, A will elute first.
Question 21.
Without using column chromatography, how will you separate a mixture of camphor and benzoic acid?
Answer:
Sublimation cannot be used as both camphor and benzoic acid sublime on heating. Therefore, a chemical method using NaHCO3 solution is used when benzoic acid dissolves leaving camphor behind. The filtrate is then cooled with dilute HCl to get benzoic acid.
Question 22.
0.12g of an organic compound containing phosphorus gave 0.22g of Mg2P207 by the usual analysis. Calculate the percentage of phosphorus in the compound.
Answer:
Mass the substance taken (w) = 0.12g
Wt. of Mg2P2O7 formed (x) = 0.22 g
222 g of Mg2P2O7contain phosphorus = 62 g.
% of phosphorus = \(\frac{62}{222} \times \frac{0.22}{0.12}\) × 100 = 51.20
Question 23.
Compare inductive & mesomeric effects.
Answer:
| Inductive effect | Mesomeric effect |
| 1. It operates in saturated gp. of compounds. | 1. It occurs in unsaturated & especially in conjugated compounds. |
| 2. It involves electrons in σ – bonds. | 2. It involves electrons in π – bonds. |
| 3. Electron pair is slightly displaced & there only partial charges are developed. | 3. The electron pair is transferred completely with the result full positive & negative charges are created. |
| 4. It is transmitted over only a quite short distance. | 4. It is transmitted from one end to the other of quite large molecules provided conjugation (i.e. delocalised orbitals) is present through which it can proceed. |
Question 24.
What is the difference between distillation, distillation under reduced pressure & steam distillation?
Answer:
| Distillation | Distillation under reduced pressure | Steam distillation |
| This is used to separate volatile liquid from non-volatile liquid or solid separately. | This is used to purify liquids that decompose at or below their boiling points. | This is used for purifying substances that are steam volatile & immiscible with water. |
Question 25.
How will you purify sugar which has impurities of sodium chloride?
Answer:
Sugar may be purified by the crystallization method. This can be purified by shaking the impure solid with hot ethanol at 345K. The sugar will dissolve whereas common salt remains insoluble. The hot solution is filtered, concentrated & allowed to cool when crystals of sugar will separate out. In this case, hot water has been used as a solvent. The purification of sugar would not have been possible since both sugar’& common salt are soluble in water.
Question 26.
Differentiate between Ionic & free radical reactions.
Answer:
| Ionic reactions | Free radical reactions |
| 1. These occur only rarely in the gas phase but mainly in a solution of polar solvents; the reaction is influenced by the polarity of the solvent. | 1. These occur in gas phases or in non-polar solvents. |
Question 27.
For each of the following compounds, write a more condensed formula & also their bond-line formula.
(b) HOCH2CH2CH2CHCH3CHCH3CH3
Answer:
Question 28.
Expand each of the following bond line formulae to show all the atoms including carbon & hydrogen.
Answer:
Answer:
Answer:
Question 29.
Explain why is (CH3) C+ more stable than CH3 C+ H2 & C+ H3 is the least stable cation?
Answer:
Hyperconjugation interaction in (CH3)3 C+ is greater than in C++ H3 C+ H2 has 9 C -H bonds. In C H3, the C -H bonds are in the nodal plane of the vacant 2p-orbital & hence cannot overlap with it.
Thus C+ H3 is least stable.
Question 30.
The choice of the solvent is of great importance in crystallizing organic substances. What are the characteristics of a suitable solvent?
Answer:
A suitable solvent must have the following characteristics;
- The impurities & pure compound must have a large difference in their solubilities.
- The pure compound must have low solubility at room temperature but high solubility at its boiling point.
- The impurity should either be insoluble at room temperature or must have high solubility so that crystallization may give a high yield.
- The solvent should have an average boiling point.
- The solvent should neither react with the compound nor with impurities.
- The solvent should not be highly inflammable.
Organic Chemistry: Some Basic Principles and Techniques Important Extra Questions Long Answer Type
Question 1.
Explain the principle of steam distillation.
Answer:
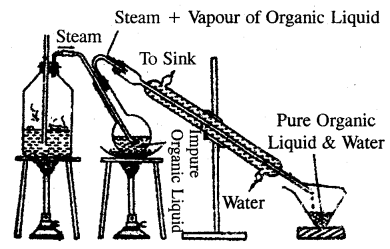
Steam distillation: The process of steam distillation is employed in the purification of substance from non-volatile impurities provided the substance itself is volatile in steam and insoluble in water.
This method is based on the facts that
- A liquid boils at a temperature when its vapour pressure becomes equal to the atmospheric pressure.
- The vapour pressure of a mixture of two immiscible liquids is equal to the sum of the vapour pressures of the individual liquids.
In the actual process, steam is continuously passed through the impure organic liquid. Steam heats the liquid and it gets practically condensed to water. After some time mixture of the liquid and water begins to boil, because the vapour pressure of the mixture becomes equal to the atmospheric pressure.
Obviously, this happens at a temperature that is lower than the boiling point of the substance or that of water. Thus an organic compound boils below its boiling points and chances of decomposition avoided. For example, a mixture of aniline (b.p 453 K) with decomposition and water (b.p. 373 K) under normal atmospheric pressure boils at 371K. At this temperature the
Steam Distillation
water boils at 371 K. At this temperature, the vapour pressure of water is 717 mm and that of aniline is 43 mm and therefore the total pressure is equal, to 760 mm. Thus in steam distillation, the liquid gets distilled at a temperature lower than its boiling point and chances of decomposition avoided. The proportion of water and liquid in the mixture that distils over is given by the relation.
\(\frac{w_{1}}{w_{2}}=\frac{P_{1} \times 18}{P_{2} \times M}\)
where w1 and w2 stand for the masses of water and liquid that distils over. P1 and P2 are vapour pressure of water and of liquid at the distillation temperature and M is the molecular mass of the liquid.
Question 2.
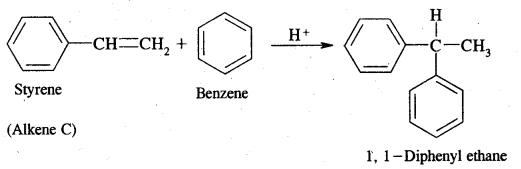
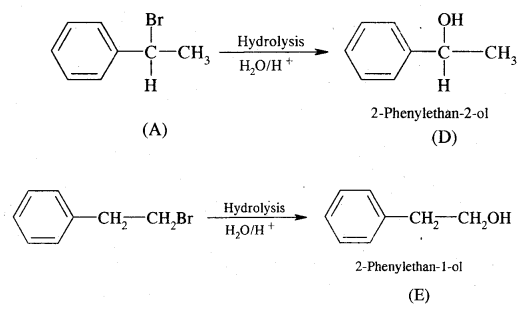
Dehydrobromination of compounds (A) and (B) yield the same alkene (c) Alkene (c) Can regenerate (A) and (B) by the addition of HBr in the presence and absence of peroxide respectively. Hydrolysis of A and B give isomeric products (D) and (E) respectively. 1, 1-Diphenyl ethane is obtained on the reaction of (C) of benzene in the presence of H+ ions. Give structures of A to E with reactions.
Answer:
Alkene (C) on reaction with benzene in the presence of H+ ions gives 1, 1-Diphenyl ethane. Therefore C must be styrene as depicted below
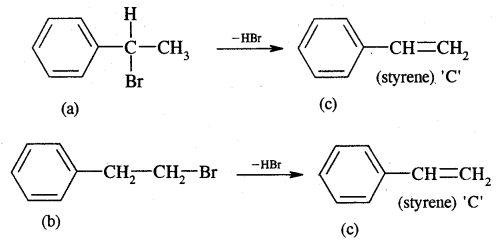
Now dehydrobromination of A and B give the same alkene C, i.e.,
styrene.
∴ A and B must be isomeric alkyl bromide.
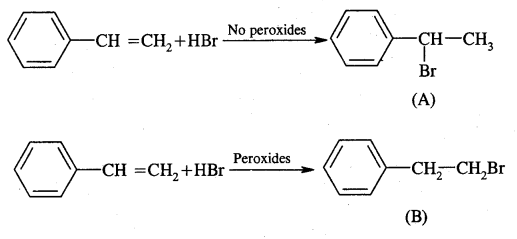
A and B can be obtained by the addition of HBr in the presence and absence of peroxide to styrene.
Hydrolysis of A and B give isomeric alcohols (D) & (E) as
Question 3.
What are reaction intermediates? How are they generated by bond fission?
Answer:
The species which are generated as a result of bond fission are called reaction intermediates. The important reaction intermediates are:
1. Free Radicals: A free radical may be defined as an atom or group of atoms having an impaired electron. These are obtained as a result of homolytic fission of covalent bonds.

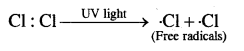
These free radicals are neutral particles, extremely transient, (short-lived) and highly reactive. They get consumed as soon as they are formed. They pair up their electron with another electron from wherever it is available. They occur only as a reaction intermediate. Their presence is felt in reactions, but cannot be isolated in a free state. For example dissociation of Cl2 gas in the presence of Ultraviolet light produces free radicals.
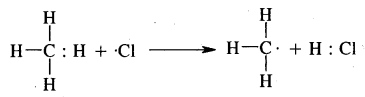
The alkyl free radicals are obtained when free radical: Cl reacts with alkanes.
Free radical may be primary, secondary, tertiary depending upon whether, one, two or three carbon atom attached to the carbon atoms carrying the odd electron.
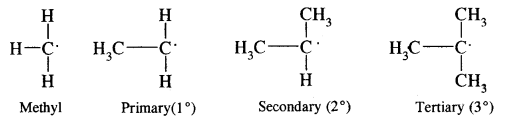
The stability is CH3 < 1° < 2° < 3°.
2. Carbocation or carbonium ion: It is defined as a group of atoms that contain positively charged carbon having only six electrons. It is obtained by heterolytic fission of a covalent bond involving a carbon atom.
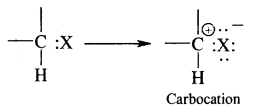
They are also classified as primary, secondary and tertiary depending upon whether one, two or three carbon atoms are attached to the carbon bearing the positive charge as:
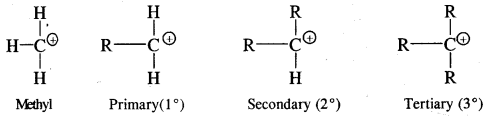
Thus the order of stability if CH3+ < 1° < 2° < 3°.
3. Carbanion: A carbanion may be defined as a species containing a carbon atom carrying a negative charge. These are generated by the atom in which the atom linked to carbon goes without the bonding electrons. As a result of this carbon acquires a negative charge. For example, the removal of hydrogen of methyl part of acetaldehyde molecule as H+ ion leaving both the electron on carbon.
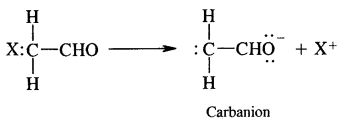
They are also very reactive species. They are also classified as primary, secondary and tertiary depending upon whether one, two or three carbon atoms are attached to the carbon atom bearing negative
charge.
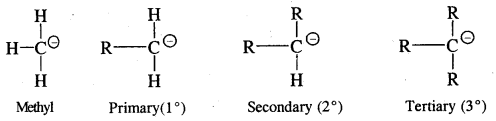
The order of stability is the reverse of free radicals and carbocations
CH3 – > 1° > 2° > 3°.
(iv) Carbenes: The carbenes are reactive neutral species in which carbon atom has six electrons in the valency shell out of which two are shared. The simplest carbene is methylene (CH2). It is formed when diazomethane is decomposed by the action of light.
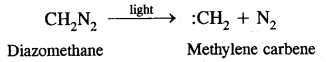
It is very reactive. It reacts with alkenes by adding to the double bond forming cyclopropane.
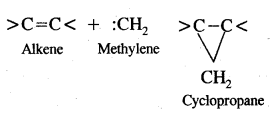
Organic Chemistry: Some Basic Principles and Techniques Important Extra Questions Numerical Problems
Question 1.
0.395 g of an organic compound by various method for the estimation of sulphur gave 0.582g of BaS04. Calculate the percentage of Sulphur.
Answer:
Mass of BaSO4 = 0.582 g
BaSO4 = S
233 = 32
233g of BaSO4 contain sulphur = 32g
0. 582 g of BaSO4 contains sulphur
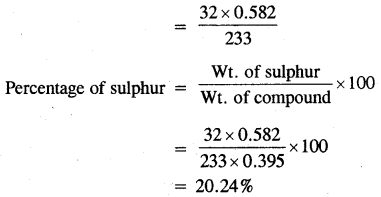
Question 2.
0.15g of an organic compound gave 0.12g of AgBr by carius method. Find the percentage of bromine in the compound.
Answer:
Mass of AgBr formed = 0.12g
188 g of AgBr contains bromine = 80g.
Therefore, 0.12g of AgBr will contain bromine
= \(\frac{80 \times 0.12}{188}\) = 0.051 g
Percentage of bromine = \(\frac{0.051}{0.15}\) × 100 = 34%
Question 3.
0.40 g of an organic compound gave 0.3g of AgBr by carius method. Find the percentage of bromine in the compound.
Answer:
Mass of compound = 0.40 g
Now, 188 g of AgBr contains bromine = 80g.
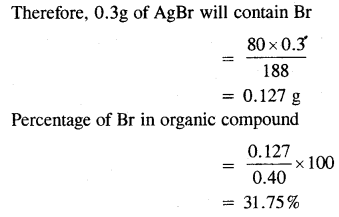
Question 4.
0.15 g of an organic compound gave 0.12g of silver bromide by the carius method. Find out the percentage of bromine in the compound.
Answer:
Here the mass of substance taken = 0.15g
Mass of AgBr formed = 0.12g
Now 1 mole of AgBr = 1 g. atom of Br
or
188 g = 80g of Br
188g of AgBr contains bromine = 80g.
Hence 0.12g of AgBr contain bromine
= \(\frac{80 \times 0.12}{188}\) = 0.051 g
Thus, 0.15g of compound contains 0.051g of Br
Percentage of Br = \(\frac{0.051}{0.15}\) × 100 = 34%
Question 5.
0.2595g of an organic compound when treated with carius method, gave 0.35g of BaSO4. Calculate the percentage of Sulphur in the compound.
Answer:
Here the mass of substance taken = 0.35g
Mass of BaSO4 ppt. formed = 0.35g
Now 1 mole of BaSO4 = 1 g. atom of Sulphur
233 g of BaSO4 = 32g of S
i. e. 233g of BaS04 contains 32g of Sulphur
Therefore 0.35g of BaSO4 will contain Sulphur
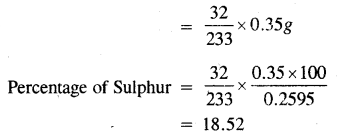
Question 6.
0.12g of an organic compound containing Phosphorus gave 0.22g of Mg2P2O7 by the usual analysis. Calculate the percentage of Phosphorus in the compound.
Answer:
Here the mass of organic compound taken = 0.12g
Mass of Mg2P2O7 formed = 0.22g .
Now 1 mole of Mg2P2O7 = 222 g of Mg2P2O7
= 62g of Phosphorus
i. e. 222g of Mg2P2O7 contains Phosphorus = 62g
Therefore 0.22g of Mg2P2O7 contains Phosphorus
= \(\frac{62}{222}\) × 0.22
Hence Percentage of Phosphorus = \(\frac{62}{222} \times \frac{0.22}{0.12}\) × 100 = 51.2
Question 7.
In a Dumas nitrogen estimation, 0.303g of an organic compound gave 50 cm3 of nitrogen collected at 300k & 715 m.m. of pressure. Calculate the percentage of nitrogen in the compound, (vapour pressure of water at 300K = 15 m.m.).
Answer:
Vapour pres1 re of the gas = 715 – 15 = 700 mm.
V1 = 50 cm3, V2 =?, P1 = 700 mm, P2 = 760 mm,
T1 = 300 K, T2 = 273 K
Applying \(\frac{\mathrm{P}_{1} \mathrm{~V}_{1}}{\mathrm{~T}_{1}}=\frac{\mathrm{P}_{2} \mathrm{~V}_{2}}{\mathrm{~T}_{2}}\)
or
V2 = \(\frac{P_{1} V_{1} T_{2}}{P_{2} T_{2}}\)
= \(\frac{700 \times 50 \times 273}{760 \times 300}\)
= 41.9 cm3
22400 cm3 of nitrogen at S.T.P. weighs = 28g.
41.9 cm3 of nitrogen at S.T.P. weighs
= \(\frac{28}{22400}\) × 41.9 = 0.0524 g
Percentage of nitrogen = \(\frac{0.0524}{0.3}\) × 100 = 17.46
Question 8.
In an estimation of Sulphur by various method, 0.2175g of a compound gave 0.5825g barium sulphate. Calculate the percentage of sulphur in the compound.
Answer:
Mass of the compound = 0.2175 g
Mass of barium sulphate = 0.5825 g
Molecular mass of BaS04 = 137 + 32 + 64 = 233 g
233 g of BaSO4 contains sulphur = 32g
0.5825g of BaSO4 contains Sulphur
= \(\frac{32}{233}\) × 0.5825g
Percentage of Sulphur = \(\frac{32}{233} \times \frac{0.5825}{0.2175}\) × 100
= 36.78
Question 9.
0.515 g of an organic compound containing Phosphorus give 0.214g of magnesium Pyrophosphate in various method for the estimation of Phosphorus. Calculate the percentage of Phosphorus in the given organic compound.
Answer:
Mass of organic compound = 0.515 g
Mass of magnesium Pyrophosphate = 0.214 g
Molar mass of Mg2P2O7 = 222 g
222 g of Mg2P2O7 obtained from Phosphorus = 62g
0.214g of Mg2P2O7 are obtained from Phosphorus
= \(\frac{62}{222}\) × 100 = 0.0597 g
Percentage of Phosphorus = \(\frac{0.0597}{0.515}\) × 100 = 11.6
Question 10.
In Duma’s method for estimation of Nitrogen 0.3g of an organic compound gave 50 mL of nitrogen collected at 300K temperature & 715 mm pressure. Calculate the percentage composition of nitrogen in the compound (Aqueous tension at 300K = 15 mm)
Answer:
Volume of nitrogen collected at 300K & 715 mm Pressure = 50 mL
Actual pressure = 715 – 15 = 700 mm
Volume of nitrogen at S.T.P = \(\frac{273 \times 700 \times 50}{300 \times 760}\) = 41.9 mL
22400 mL of nitrogen weighs = \(\frac{28 \times 41.9}{22400 g}\)
Percentage of nitrogen = \(\frac{28 \times 41.9 \times 100}{22400 \times 0.3}\) = 17.46
Question 11.
Ammonia produced when 0.75g of a substance was KJeldahlized, neutralized 30 cm3 of 0.25N H2SO4. Calculate the percentage of nitrogen in the compound.
Answer:
Mass of organic substance = 0.75g
Volume of H2SO4 used up = 30 cm3
Normality of sulphuric acid = 0.25 N 30 cm3
H2SO4 of normality 0.25 N = 30 mL of NH3 solution of normality 0.25N
But 1000 cm3 of ammonia solution of normality 1 contains 14 g of nitrogen
Therefore, 30 cm3 of 0.25N ammonia solution will contain nitrogen
= \(\frac{14}{1000}\) × 30 × 0.25
Percentage of nitrogen = \(\frac{14}{1000}\) × \(\frac{30 \times 0.25}{0.75}\) × 100 = 14