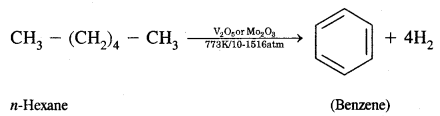Here we are providing Class 11 chemistry Important Extra Questions and Answers Chapter 13 Hydrocarbons. Chemistry Class 11 Important Questions are the best resource for students which helps in Class 11 board exams.
Class 11 Chemistry Chapter 13 Important Extra Questions Hydrocarbons
Hydrocarbons Important Extra Questions Very Short Answer Type
Question 1.
Give different isomers of C4H10 with their I.U.P.A.C. names.
Answer:

Question 2.
Give the I.U.P.A.C. name of the lowest molecular weight alkane that contains a quaternary carbon.
Answer:
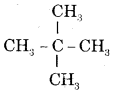
It is and its I.U.P.A.C. name is 2, 2-Dimethylpropane.
Question 3.
Which of the following has the highest boiling point?
(i) 2-methylpentane
(ii) 2, 3 – dimethylbutane
(iii) 2, 2-dimethylbutane.
Answer:
(i) 2—methyl pentane has the largest surface area and hence has the highest boiling point.
Alkene-reactions-cheat-sheet-summary-for-organic-chemistry-reactions.
Question 4.
Give the structure of the alkene (C4H8) which adds on HBr in the presence and in the absence of peroxide to give the same product C4H9Br.
Answer:
2-Butene with structure CH3 – CH = CH — CH3 being symmetrical gives the same product, i.e., 2-bromobutane CH3 CH (Br) CH2CH3.
Question 5.
How will you separate propene from propyne?
Answer:
Bypassing the mixture through ammoniacal AgNO3 solution when propyne reacts while propene passes over.
Question 6.
Name two reagents that can be used to distinguish \ between ethene and ethyne.
Answer:
Tollen’s reagent | Ammoniacal AgNO3 | and amm. CuCl solution.
Question 7.
How will you detect the presence of unsaturation in an organic compound?
Answer:
Either by Baeyer’s reagent

or by Br, in CC14.
Question 8.
Arrange the following In order of increasing volatility: gasoline, kerosene, and diesel.
Answer:
Diesel, kerosene, gasoline.
Question 9.
Arrange the following: HCl, HBr, HI, HF in order of decreasing reactivity towards alkenes.
Answer:
HF, HCl, HBr, HI.
Question 10.
Out of ethylene and acetylene which is more acidic and why?
Answer:
Acetylene. Ethylene and acetylene have sp2, sp hybridized C atoms respectively. Due to the 50% S character of the C – H bond of acetylene rather than the 33% S-Character of the C – H bond in ethene, acetylene is more acidic.
Question 11.
Write the structure of the alkene which on reductive ozonolysis gives butanone and ethanal.
Answer:
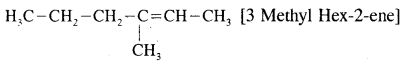
Question 12.
Write the I.U.P.A.C. names of
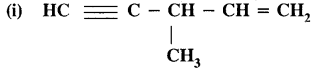
Answer:
3-methylpent-l— en—4-yne.
(ii) CH2 = CH – CH (CH3) – CH = CH – CH – CH2,
Answer:
3-methylhept-1, 4, 6—triene.
Question 13.
Draw the structures of the following:
(i) Dicyclopropyl methane
Answer:
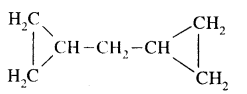
(ii) 2-methyl-3—isopropyl heptane.
Answer:
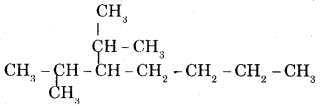
Question 14.
What effect the branching of an alkane has on its boiling point?
Answer:
Branching decreases the boiling point.
Question 15. Which of the following polymerizes most readily?
(i) Acetylene
(ii) Ethene
(iii) Buta —1, 3—diene
Answer:
(iii) Buta—1, 3—diene polymerizes most readily, being more reactive.
Question 16.
Arrange the following in increasing order of their release of energy on combustion
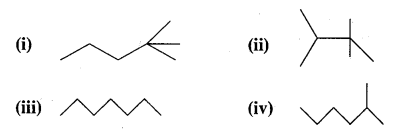
Answer:
The more the number of C atoms having maximum hydrogen hydrogens, i.e., CH3 groups, the greater is the heat of combustion. Thus the increasing order of heat of combustion is (iii) < (iv) < (i) < (ii).
Question 17.
Arrangement of the following set of compounds in order of their decreasing relative reactivity with an electrophile E+.
(i) Chloro benzene, 2, 4 —dinitrochlorobenzene p—nitrochlorobenzene.
(ii) toluene, p – CH3 – C6H4 – CH3, p – CH3 – C6H4 – NO2, p – O2N – C6H4 – NO2
Answer:
Electron-donating groups increase the reactivity towards an electrophile E+, while electron-withdrawing groups decrease the reactivity. Thus
(i) Chlorobenzene > p — nitrochlorobenzene > 2. 4—di nitrochlorobenzene.
(ii) p – CH3 – C6H4 – CH3 > toluene > p – CH3 – C6H4 – NO2 > p – O2N – C6H4 – NO2.
Question 18.
What is the order of reactivity of halogen and alkyl groups in the dehydrohalogenation of alkyl halides to give alkenes?
Answer:
- Halogens: Iodine > Bromine > Chlorine
- Alkyl group: Tert > secondary > primary.
Question 19.
What products are formed when zinc reacts with
(i) vicinal C2H4Br2 and
Answer:
CH2Br – CH2Br + Zn → CH2 = CH2 + ZnBr2.
(ii) CH3CHBr – CH2Br.
Answer:
CH3 – CHBr – CH2Br + Zn → CH3 — CH = CH2 + ZnBr2.
Question 20.
What does L.P.G. stand for?
Answer:
L.P.G. stands for liquefied petroleum gas.
Question 21.
What do the terms (i) CNG and LPG stand for?
Answer:
- CNG: Compressed natural gas
- LPG: Liquefied Petroleum gas.
Question 22.
What are sources to obtain:
(i) LPG
Answer:
LPG is obtained by the fractional distillation of petroleum.
(ii) CNG?
Answer:
CNG is obtained by the fractional distillation of coal tar.
Question 23.
Write down the structures and names of all isomers with a molecular formula of C5H12.
Answer:
- (i) CH3 — CH2 — CH2 — CH2 — CH3 is n-Pentane or Pentane.
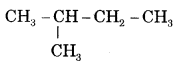
is iso-pentane or 2-Methylbutane.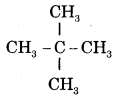
is neo-pentane or 2, 2-Dimethylpropane.
Question 24.
The sodium salt of which acid will give ethane on heating with soda-lime? Give reaction.
Answer:
Propionic acid.
![]()
Question 25.
What products are obtained by the acidic dehydration of
(i) Ethanol
Answer:
Ethene
(ii) Propan – 2—ol.
Answer:
Propene.
Question 26.
Out of cis-2-butene and trans-2-butene which is polar and which one is non-polar?
Answer:
Cis – 2 – butene is polar (μ = 0.33 D) and trans – 2 – butene is non-polar (μ = 0). .
Question 27.
Arrange the following in the decreasing order of acidic character.
(i) C2H4, C2H6, C2H2
Answer:
H – C ≡ C H > H2C = CH2 > H3C – CH3
(ii) CH3 – C = CH, C2H2, CH3 – C = C – CH3
Answer:
HC = CH > CH3 — C ≡ CH > > CH3 – C ≡ C – CH3.
Question 28.
Name two industrial sources of hydrocarbons.
Answer:
- Petroleum
- Coal.
Question 29.
Arrange the following in the increasing order of C – C bond length C2H6, C2H4, C2H22.
Answer:
C2H2 < C2H4 < C2H6.
Question 30.
What type of hydrocarbons is present in high octane gasoline?
Answer:
Branched-chain aliphatic and/or aromatic hydrocarbons.
Question 31.
What are the chief constituents of light oil fraction?
Answer:
Benzene, toluene, and xylenes.
Question 32.
Which of the following shows geometrical isomerism?
(i) CHCl = CHCl
(ii) CH2 = CCl2
(iii) CCl2 = l. Give the structures of cis-and transforms.
Answer:
(i) HC (Cl) = CH (Cl);

Question 33.
Name the product formed when methyl bromide is treated with sodium and ether.
Answer:
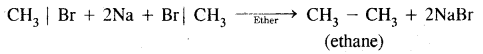
Question 34.
Which of the following shows geometrical isomerism?
But-1—ene or but—2—ene.
Answer:
But-2—ene CH3 – CH = CH – CH3.
Question 35.
Write the structure and I.U.P.A.C. name of Acetonitrile.
Answer:
Acetonitrile is CH33 CN. Its I.U.P.A.C. name is Ethane nitrile.
Question 36.
Why does carbon has a larger tendency of catenation than silicon although they have the same number of valance electrons?
Answer:
It is due to the smaller length of the C – C bond which is stronger (335 kJ mol-1) than the Si-Si bond (225.7 kJ mol-1).
Question 37.
Give name atm structure to the first organic compound synthesized in the laboratory.
Answer:
Urea
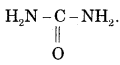
Question 38.
Benzene is highly unsaturated, yet it does not give usual addition reactions readily. Why?
Answer:
Benzene is highly unsaturated, yet, resonance imparts extra-stability to benzene and it does not give additional reactions.
Question 39.
What is Lindlar’s catalyst? What is it used for?
Answer:
Pd/BaSO4 poisoned with quinoline. It is used for the partial reduction of alkynes to cis-alkenes.
Question 40.
How will you detect the presence of unsaturation in an organic compound?
Answer:
Either by Baeyer’s reagent or by Br2 in CCl4. The color is discharged.
Question 41.
How can ethylene be converted to ethane?
Answer:
![]()
Question 42.
Arrange the following in increasing order of C — C bond length C2H6, C2H4, C2H2.
Answer:C2H2 < C2H44 < C2H6.
Question 43.
What type of hybridization is involved in
(i) planar and
Answer:
sp2
(ii) linear molecules?
Answer:
sp.
Question 44.
Name the chain isomer of C55H12 which has a tertiary hydrogen atom.
Answer:
2-Methyl butane (CH3), CHCH2CH3.
Question 45.
What type of isomerism is shown by butane and isobutane.
Answer:
Chain or nuclear isomerism,
Question 46.
What do you mean by cracking?
Answer:
The thermal decomposition of higher hydrocarbons into lower hydrocarbons in the presence or absence of a catalyst is called cracking.
Question 47.
Complete the reaction HC ≡ CH
![]()
Answer:
![]()
Question 48.
Out of Octane and is heptane, which has a lower octane number?
Answer:
n-Octane.
Question 49.
What are the main components of LPG?
Answer:
Butane and isobutane.
Question 50.
Write a chemical reaction to illustrate the Saytzeffs rule.
Answer:
Hydrocarbons Important Extra Questions Short Answer Type
Question 1.
The following organic compounds are known by their common names
(i) Neopentane
Answer:
Neopentane is
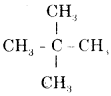
& its h.U.P.A.C. name is 2, 2—dimethyl propane.
(ii) Acetone
Answer:
Acetone is
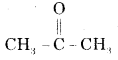
and its I.U.P.A.C. name is Propanone.
(iii) Vinyl chloride
Answer:
Vinyl chloride is CPU = CH — Cl and its I.U.P.A.C. name are chloroethene.
(iv) Tert butyl alcohol. Write their structural formulae and I.U.P.A.C. names.
Answer:
Tert; butyl alcohol
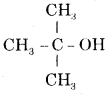
and its I.U.P.A.C. name is 2-methyl propan-2-ol.
Question 2.
What are the various products expected when propane reacts with fuming nitric acid?
Answer:
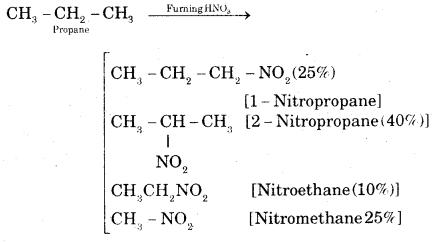
Question 3.
How will you convert methane into
(i) Methanol
Answer:
Conversion of methane into methanol:
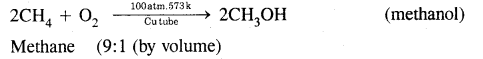
(ii) Methanal.
Answer:
![]()
Question 4.
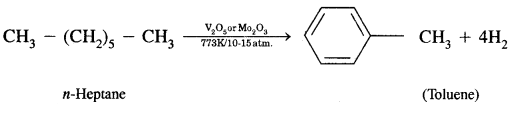
What is aromatization? How will you convert ^hexane into benzene?
Answer:
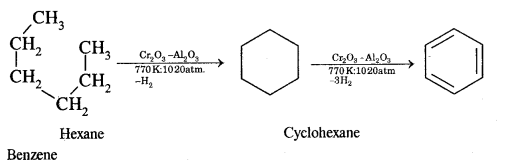
Aromatization. It is the process that involves cyclization, isomerization, and dehydrogenation with the application of heat and catalyst to convert alkanes containing six or more carbon atoms into aromatic hydrocarbons.
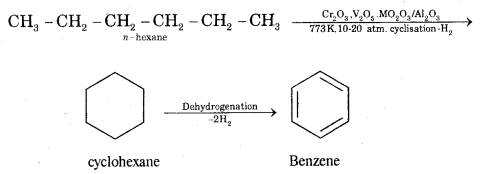
Question 5.
Give the different conformations of ethane with their
(i) Sawhorse representation and
(ii) Newmann Projection formulae.
Answer:
Sawhorse representation Newmann projection models
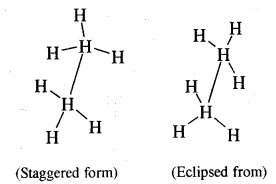
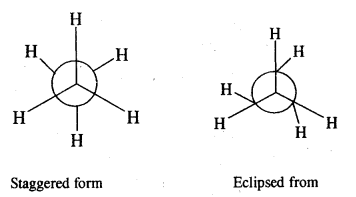
Question 6.
What are the relative stabilities of different conforma¬tions of ethane? Is it possible to isolate these at room temperature?
Answer:
The staggering form of ethane is more stable than the eclipsed form because the force of repulsion between hydrogen atoms on adjacent C atoms is minimum. The energy difference between the staggered form and eclipsed form of ethane is just 12.55 kJ mol-1. Therefore, it is not possible to separate these two forms of ethane at room temperature.
Question 7.
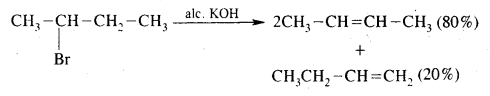
What is Saytzeff Rule? What are the expected products when 2-Bromobutane is dehydrohalogenation with ale. KOH?
Answer:
Saytzeff Rule. Whatever two alkenes are theoretically possible during a dehydrohalogenation reaction, it is always the more highly substituted alkene that predominates.
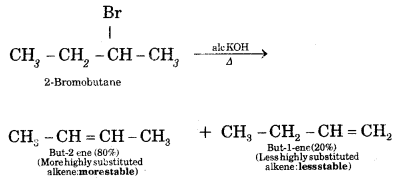
Question 8.
What is the order of reactivity of H2C = CH2, (CH3)2, H2C = CH2, CH3 – CH = CH2, CH3 – CH = CH – CH3, (CH3)2 C = C (CH3)2, (CH3)2 C = CH CH3 towards electrophilic addition reactions?
Answer:
The order of reactivity of the above alkenes towards electrophilic addition reactions decreases in the order.
(CH3)2 C = C (CH3)2 > (CH33)2 C = CH CH3 > (CH3)2 C = CH2 > CH3 CH – CH – CH3 > CH3 – CH = CH, > CH2 = CH2.
Question 9.
Define Markownikov rule. Explain it with an example.
Answer:
Markownikov rule states. The negative part of the addendum adding to an unsymmetric alkene goes to that C atom of the double bond which is attached to a lesser number of C atoms.
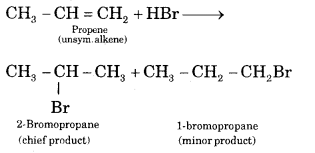
Question 10.
What is the Peroxide effect/Kharasch Effect? Illustrate with an example.
Answer:
In the presence of peroxides such as benzoyl peroxide, the addition of HBr (but not of HCl or HI) to an unsymmetrical alkene takes place contrary to the Markownikov rule. This is known as the peroxide/Kharsch effect.
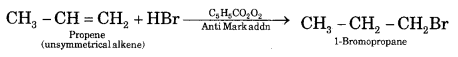
Question 11.
An alkene with the molecular formula C7H14 gives propanone and butanal on ozonolysis. Write down its structural formula and its I.U.P.A.C. name.
Answer:
The structures of the compounds on ozonolysis of C7H14 and
![]()
Remove the oxygen atoms and connect them by a double bond, the structure of the alkene is
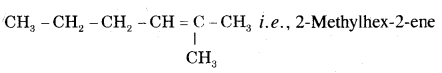
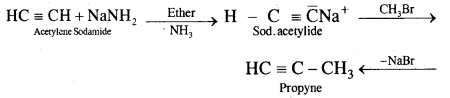
Question 12.
How will you prepare propyne and I-Butyne from acetylene;?
Answer:
(i) Preparation of propyne from acetylene
(ii) Preparation of 1-butyne from acetylene
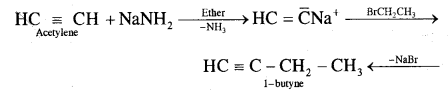
Question 13.
Describe a method to distinguish between ethane, ethene, ethyne.
Answer:
(i) Ethene (C2H4) and ethyne (C2H2) decolorize bromine in carbon tetrachloride whereas ethane (C2H6) does not.
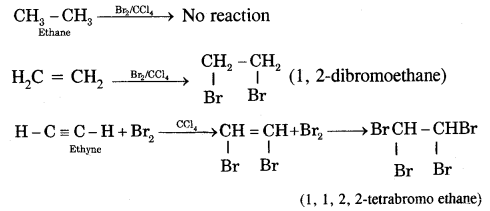
(ii) Ethyne (and not ethane, ethene) reacts with ammoniacal AgNO3 (Tollen’s reagent) to form white precipitates.
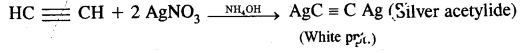
Question 14.
Give the mechanism of an electrophilic addition of chlorine into propene.
Answer:
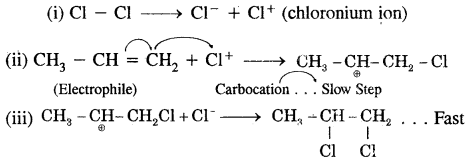
Cl4 (chloronium ion) formed by the heterolytic fission of Cl2 in step (i) being an electrophile attacks propene in (ii) step, propene undergoes electrometric effect combined with + I effect to form carbocation which, is a slow step. In (iii) step Cl ion being a. nucleophile attacks carbocation and forms the product. It is a fast step.
Question 15.
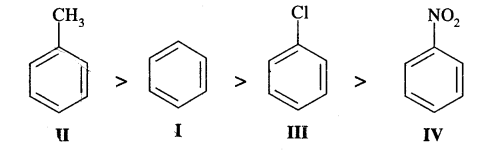
Identify the correct order of reactivity in electrophilic substitution reactions of the following compounds: [I.I.T. 2002]
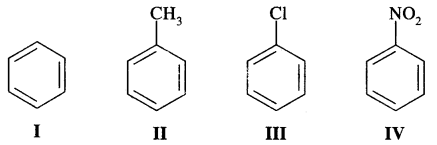
Answer:
— NO2 group in structure IV is an electron attracting group, it deactivates the benzene ring largely towards electrophilic substitution reactions. Cl group in III is also a deactivating group, but its deactivation is lower too — NO2, where—CH3, a group in II is an electron-releasing group and so activates the benzene ring towards electrophilic substitution. Therefore, the order of reactivity is
Question 16.
What is meant by (i) delocalization
Answer:
Delocalisation: Delocalisation means that pairs of 7t electrons extend over 3 or more atoms. They belong to the whole molecule. For example, 6n electrons present in benzene are delocalized and are spread on the whole of the ring and this imparts extra stability to the molecule.
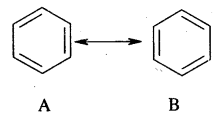
(ii) resonance energy.
Answer:
Resonance energy: The difference between the energy of the most stable contributing/canonical structural and the energy of the resonance hybrid is known as resonance energy. In the case of benzene, the resonance hybrid has 147 kJ mol-1 than either A or B below. Thus resonance energy of benzene is 147 kJ mol-1.
Question 17.
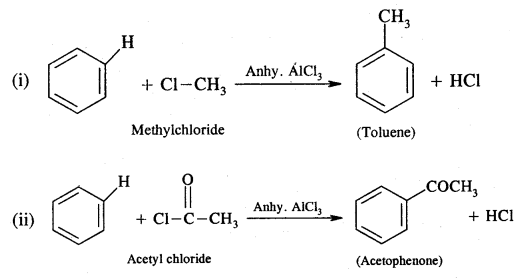
Describe Friedel’s craft reaction with suitable examples.
Answer:
When an alkyl or acid halide is treated with benzene or its derivative in the presence of anhydrous AlCl3 as a catalyst, we got alkyl or acyl benzene.
Question 18.
Classify the following hydrocarbons into alkanes, alkenes, alkynes, and arenes.
(i) (CH3)4C
Answer:
Alkane
(ii) C2H2
Answer:
Alkyne
(iii) C3H6
Answer:
Alkene
Answer:
Arene.
Question 19.
Write all possible structures of C5H8 and give their I.U.P.A.C. names.
Answer:
Structural isomers of C5H8 (Pentyne) are
(i) CH3 – CH2 – CH2 – C ≡ CH (Pent-l-yne)
(ii) CH3 – CH2 – C ≡ C – CH3 (Pent-2-yne)
(iii) 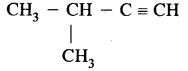
(3-Methylbut-l-yne)
Question 20.
How does ethylene undergo polymerization? What is the use to which the polymer obtained is put?
Answer:
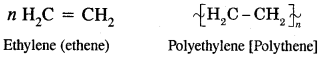
Low-density polythene [LDPE] and high-density polyethylene [HDPE] as used for the manufacture of plastic bags, squeeze bottles, refrigerator dishes, toys, pipes, radio, and T.V. cabinets, etc.
Question 21.
What is the action of water on
(i) Ethyne
(ii) Propyne? Name the end products obtained.
Answer:
(i) Action of water on ethyne: When ethyne is warmed with dilute H2SO4 at 333K and H8SO4 as catalyst ethanol is obtained.
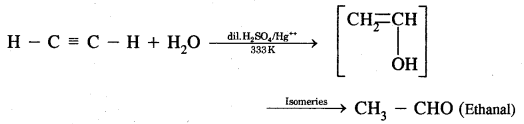
(ii) Action of water on Propyne CH3 — C ≡ CH + H2O
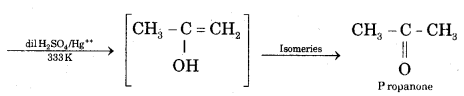
Question 22.
Propene reacts with HBr to give Isopropyl bromide but does not give n-propyl bromide. Why?
Answer:
The addition of unsymmetrical addendum (HBr) to unsymmetrical olefines (CH3CH = CH2) takes place according to Markownikov rule, the negative part of reagent (i.e. Br-) adds on the carbon atom having a minimum number of hydrogen atoms. Hence Isopropyl bromide will be formed.
![]()
Question 23.
An oxidizing agent is needed in the iodination of methane but not in the chlorination or bromination. Give reason.
Answer:
In the iodination of methane, H — I is also formed as the product with iodomethane, since it is a strong reducing agent, it reduces iodomethane back to methane & makes the reaction reversible. In order to destroy HI, an oxidizing agent like HIO3 (or HNO3) is needed. But HCl& HBr formed in the chlorination & bromination reactions of methane & not in a position to react with the monosubstituted products (CH3Cl & CH3Br) since they are comparatively weak reducing agents.
Therefore, no oxidizing agent is needed for these reactions.
CH4 + I2 ⇌ CH3I + HI
5HI + HIO3 → 3H2O + 3I2
Question 24.
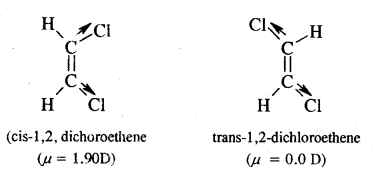
The dipole moment of trans 1, 2-chloroethene is less than the cis isomer. Explain.
Answer:
The structure of the trans isomer is more symmetrical to the cis isomer. In the trans isomer, the dipole moments of the polar C—Cl bonds are likely to cancel with each other & the resultant dipole moment of the molecule is nearly zero. But in the cis isomer, these do not cancel. Therefore, the cis isomer has a specific moment but is zero in the case of the trans isomer.
Question 25.
Write l.U.P.A.C. names of the products obtained by addition reactions of HBr to hex-l-ene.
(a) In the absence of Peroxide
Answer:
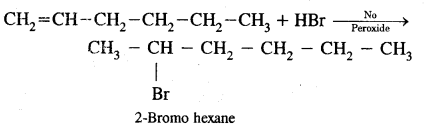
(b) In the presence of Peroxide.
Answer:
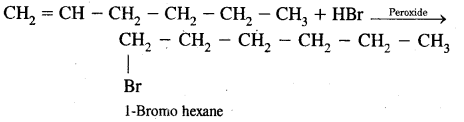
Question 26.
How will you distinguish between the following:
(a) Butyne-l & Butyne-2 ,
Answer:
Butyne-1 having an acetylene hydrogen atom will give white ppt. With ammoniacal silver nitrate & red ppt. with ammonical cuprous chloride. On the other hand, butyne-2-having no acetylenic hydrogen atom does not respond to either of the two reagents.
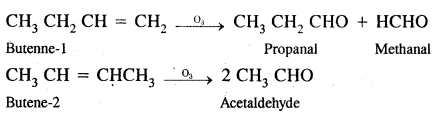
(b) Butene-1 & Butene-2
Answer:
Butene-1 & butene-2 can be distinguished either by ozonolysis or by oxidation with acidic KMnO2 solution with which they give different carbonyl compounds.
Question 27.
Alkynes are less reactive than alkenes towards addition reaction even though they contain 2-7t bond. Give reason.
Answer:
This is due to
- greater electronegativity of sp-hybridized carbon of alkynes than sp2 hybridized carbon atoms of alkenes which holds the π-electrons of alkynes more tightly and
- greater delocalization of π-electrons in alkynes (because of the cylindrical nature of their n electron cloud) than in alkenes. As a result, n electrons of alkynes are less easily available for addition reactions than those of alkenes.
Consequently, alkynes are less reactive than alkenes towards addition reactions.
Question 28.
Why do addition reactions occur more readily with alkenes & alkynes than with aromatic hydrocarbons?
Answer:
The energy gained by forming two sigma bonds (of four sigma bonds) more than compensates for the loss of one or two n bonds when addition occurs to an alkene or alkyne. However, in aromatic hydrocarbons, the aromatic ring is specially stabilized by the delocalization of n electrons about the ring.
It, therefore, requires substantial activation energy to cause the loss of its aromatic character. The most usual reaction in arenes is thus substitution rather than addition, since substitution does not result in loss of aromatic character.
Question 29.
A Hydrocarbon A, adds one mole of hydrogen in presence of platinum catalyst from n-Hexane. When A is oxidized vigorously with KMnO4, a single carboxylic acid, containing three carbon atoms is isolated. Give the structure of A & explain.
Answer:
- Since hydrocarbon A adds one molecule of H2 in presence of platinum to form n-hexane. A must be a hexene.
- Since A on vigorous oxidation with KMnO4 gives a single carboxylic acid containing three carbon atoms, therefore, A must be asymmetrical hexene i.e. hex-3-ene.
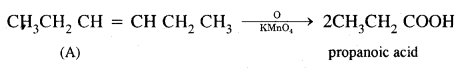
Thus, the given hydrocarbon A is hex-3-ene.
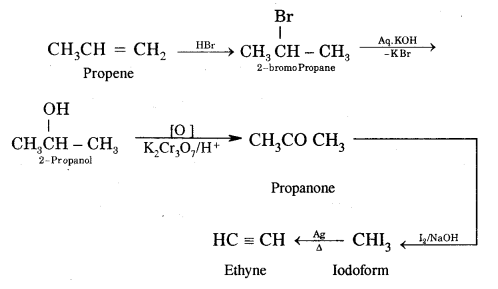
Question 30.
How would you carry out the following conversion?
Answer:
Propene to Ethyne
Hydrocarbons Important Extra Questions Long Answer Type
Question 1.
How would you convert the following compounds to benzene?
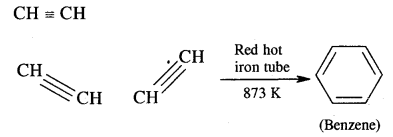
(i) Acetylene
Answer:
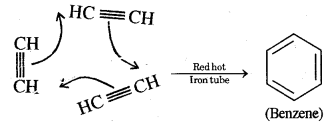
Acetylene into benzene. Ethyne (Acetylene) in passing through a red hot iron tube at 873 K undergoes cyclic polymerization as shown below.
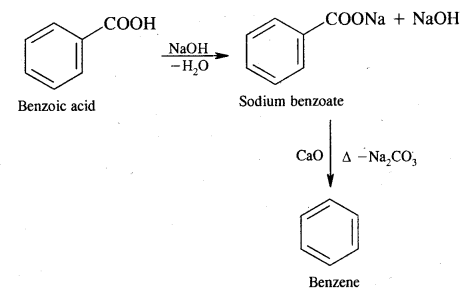
(ii) Benzoic acid
Answer:
Benzoic acid into benzene
(iii) Hexane
Answer:
Hexane into benzene
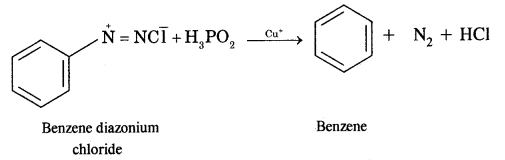
(iv) Benzene diazonium chloride
Answer:
Benzene diazonium chloride into benzene
Question 2.
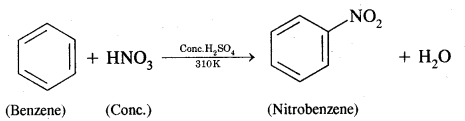
How will you convert benzene into
(i) Nitrobenzene
Answer:
Benzene into Nitrobenzene
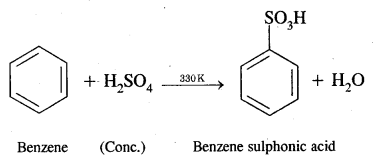
(ii) Benzene sulphonic acid
Answer:
Benzene into benzene sulphonic acid
(iii) Toluene
Answer:
Benzene into Toluene
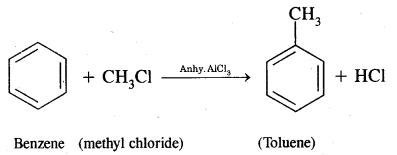
(iv) Acetophenone?
Answer:
Benzene into Acetophenone
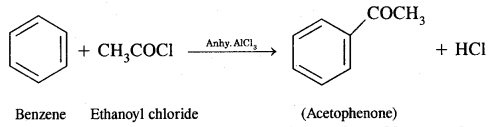
Question 3.
What is the mechanism of nitration of benzene?
Answer:
Nitration of benzene. It is carried out by treating benzene with a mixture of cones. HNO3+ Cone. H2SO4. The various steps involved are:
Step I: Generation of an electrophile, i.e., NOt (nitronium ion)
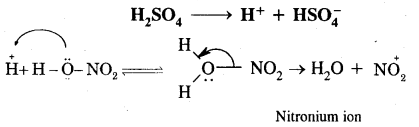
Step II: Formation of complex or carbocation intermediate
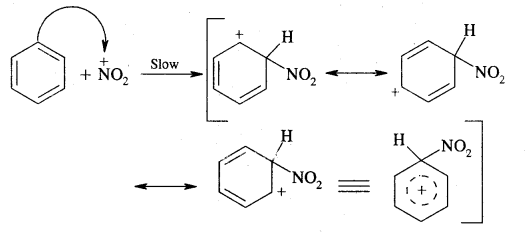
This step is slow and hence is the rate-determining step of the reaction.
Step III: Loss of a proton from the carbocation intermediate
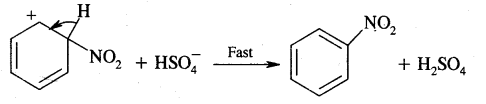
This step is fast and does not affect the rate of the reaction.
Question 4.
(a) What is the general formula of Alkynes?
Answer:
The general formula of alkynes ¡s CnH2n-2.
(b) Give the I.U.P.A.C. names and structure of all alkynes having the molecular formula C2H8.
Answer:
C5H8 has the following isomers.
(i) CH3 — CH2 — CH2 — C ≡ CH Pent-1-yne
(ii) CH3 CH, C ≡ C — CH3 Pent-2-yen
(iii) 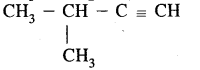
3-Methyl but-1-yen
(c) Give any two methods for preparing acetylene.
Answer:
Acetylene can be prepared by the following two methods
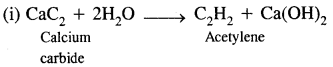
(ii) By dehydrohalogenation of dihaloalkanes
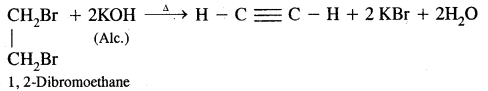
(d) Discuss any three chemical properties of acetylene.
Answer:
(i) Addition of Hydrogen:
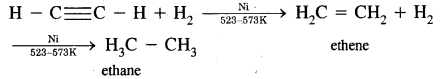
(ii) On oxidation with alkaline KMnO4, it gives oxalic acid.
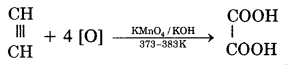
(iii) When acetylene is passed through a red hot iron tube, it trickeries to give benzene
Question 5.
(a) Write structures of different chain isomers of alkanes corresponding to the molecular formula C6H14. Also, write their I.U.P.A.C. names.
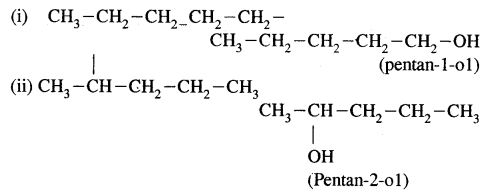
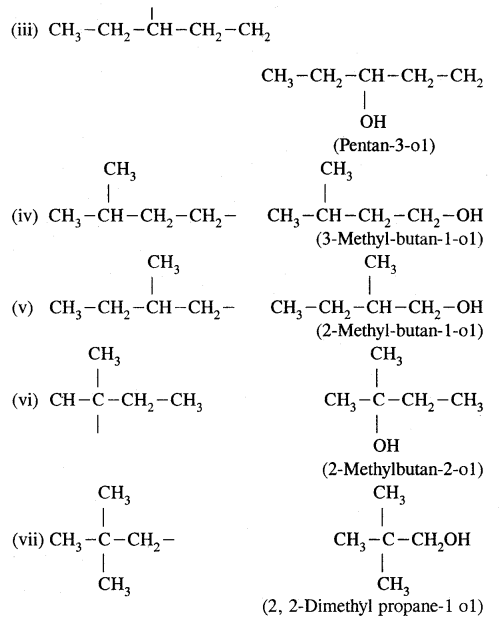
(b) Write structures of different isomeric alkyl groups corresponding to the molecular formula C5H11. Write IUPAC names of alcohols obtained by attachment of —OH groups at different carbons of the chain.
Answer:
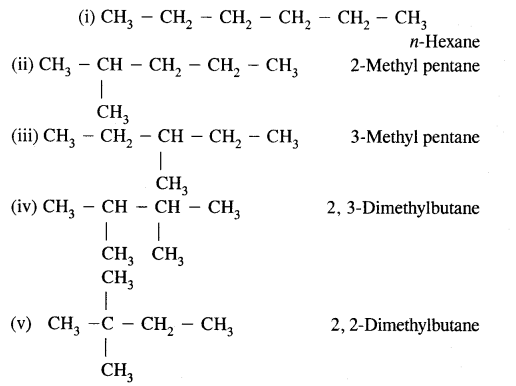
(b) Structures of – C5H11 group Corresponding alcohols
Question 6.
How will you prepare Alkanes by
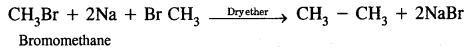
(i) Wurtz reaction
Answer:
Methods of preparation of Alkanes
Wurtz reaction. Alkyl halides on treatment with sodium in dry ether give higher alkanes, preferably containing an even number of carbon atoms.
(ii) Decarboxylation of sodium salts of fatty acids
Answer:
Sodium salts of fatty acids on heating with-soda lime (a mixture of NaOH and CaO) give alkanes containing one carbon atom less than the carboxylic acid. The process of elimination of carbon dioxide from a carboxylic acid is known as Decarboxylation
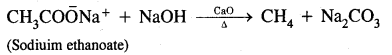
(iii) Kolbe’s electrolytic method.
Answer:
Kolbe’s electrolytic method. An aqueous solution of sodium or potassium salt of a carboxylic acid on electrolysis gives alkanes containing an even number of carbon atoms at the anode.

The probable mechanism for the reaction is
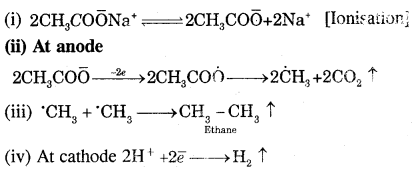
(b) How do alkanes udergo:
(i) substitution reactions with halogens
Answer:
Substitution reactions with Halogens. The order of reactivity of halogens is F2 > Cl2 > Br2 > I2
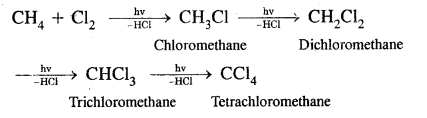
Bromination is similar. With fluorine, the reaction is too violent to be controlled. Iodination is very slow and a reversible reaction.
It can proceed in the presence of oxidizing agents like HNO3, HIO3.
CH4 + I2 ⇌ CH3I + HI
HIO3 + 5HI → I3 + 3H2O
Halogenation of alkanes proceeds via a free-radical mechanism which consists of three steps.
- Chain initiation step
- Chain propagation step
- Chain termination step
1. Chain initiating step. Cl2 undergoes hemolysis in the presence of heat and light.
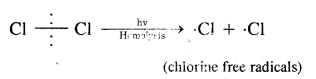
2. Chain propagation step
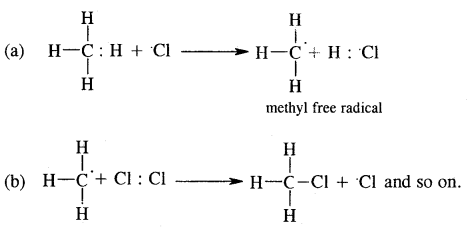
3. Chain termination step
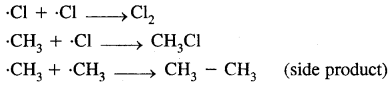
(ii) oxidation by combustion
Answer:
Oxidation of alkanes by combustion: Alkanes on heating in the presence of air or dioxygen are completely oxidized to carbon dioxide and water with the evolution of a large amounts of heat.
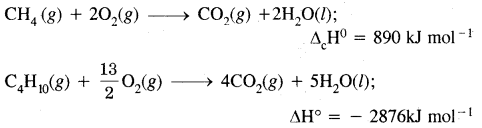
In the presence of an insufficient amount of air or dioxygen, carbon black is formed.
![]()
(iii) Isomerisation
Answer:
Isomerization reactions of alkanes, n-Alkanes on heating in the presence of anhydrous aluminum chloride and hydrogen chloride gas isomerize to branched-chain alkanes.
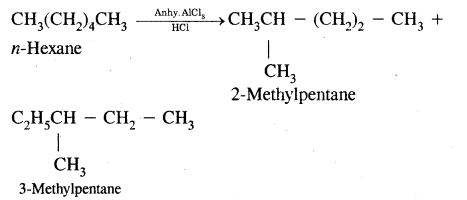
(iv) Aromatisation.
Answer:
Aromatisation. n-Alkanes having six or more C atoms on heating to 773 K at 10-20 atmospheric pressure in. the presence of oxides of vanadium, molybdenum get dehydrogenated and cyclized. This reaction is called aromatization or reforming.