Here we are providing Class 12 Chemistry Important Extra Questions and Answers Chapter 10 Haloalkanes and Haloarenes. Class 12 Chemistry Important Questions are the best resource for students which helps in Class 12 board exams.
Class 12 Chemistry Chapter 10 Important Extra Questions Haloalkanes and Haloarenes
Haloalkanes and Haloarenes Important Extra Questions Very Short Answer Type
Question 1.
Define ambident nucleophile with an example. (CBSE Delhi 2019)
Answer:
The nucleophile which has two sites through which it can attack is called ambident nucleophile, for example, nitrite ion (NO2–). It can attack either through 0 (-O– -N = O) or through N
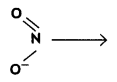
Question 2.
What happens when bromine attacks CH2 = CH – CH2 -C ≡ CH? (CBSE AI2012)
Answer:
The Colour of bromine gets discharged.
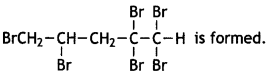
Question 3.
What happens when CH3 – Br is treated with KCN? (CBSE Delhi 2013)
Answer:
Ethane nitrile is formed.
CH3Br + KCN → CH3C ≡ N + KBr (Ethane nitrile)
Question 4.
Write the IUPAC name of
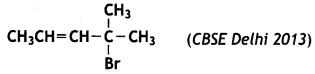
Answer:
4-Bromo-4-methylpent-2-ene
Question 5.
What happens when ethyl chloride is treated with aqueous KOH? (CBSE Delhi 2013)
Answer:
Ethyl alcohol is formed.
CH3CH2Cl + KOH(aq) → CH3CH2OH + KCl Ethyl alcohol
Question 6.
Write the IUPAC name of (CH3)2CH.CH(Cl)CH3. (CBSE Delhi 2013)
Answer:
2-Chloro-3-methyl butane
Question 7.
Which compound in the following pair undergoes a faster SN1 reaction? (CBSE Delhi 2013)
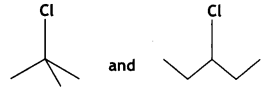
Answer:
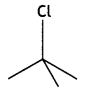
Question 8.
Write the IUPAC name of the following compound: (CBSE AI 2013)
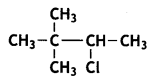
Answer:
3-Chloro-2, 2-dimethylbutane
Question 9.
Which aerosol depletes the ozone layer? (CBSE AI 2013)
Answer:
Chlorofluoro compounds of methane (CCl2F2) called freons.
Question 10.
Write the IUPAC name of the following compound: (CBSE AI 2013)
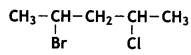
Answer:
2-Bromo-4-chloropentane
Question 11.
Why is CH2 = CH – CH2 – Cl more easily hydrolysed than CH3 – CH2 – CH2 – Cl? (CBSE AI 2019)
Answer:
CH2 = CH – CH2 – Cl gives allylic carbocation as intermediate, which is resonance stabilised.
![]()
Therefore, CH2 = CH – CH2 – Cl is more readily hydrolysed.
Question 12.
Write the IUPAC name of the following compound:
CH2 = CHCH2Br (CBSE 2011)
Answer:
3-Bromoprop-1-ene
Question 13.
Write the IUPAC name of the following compound: (CH2 )3CCH2Br (CBSE 2011)
Answer:
1 -Bromo 2,2-dimethylpropane
Question 14.
Write the IUPAC name of the following: (CBSE 2012)
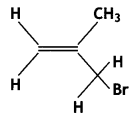
Answer:
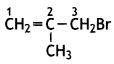
3-Bromo- 2-methylpropene
Question 15.
Identify the chiral molecule in the following pair: (CBSE 2014)
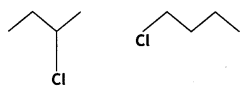
Answer:
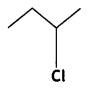
2-Chlorobutane is a chiral molecule.
Question 16.
Which would undergo SN2 reaction faster in the following pair and why? (CBSE Delhi 2015)

Answer:
CH3CH2Br would undergo SN2 reaction faster because of less steric hindrance.
Question 17.
Out 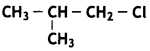 and
and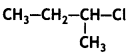 which is more reactive towards SN1 reaction and why? (CBSE Delhi 2016)
which is more reactive towards SN1 reaction and why? (CBSE Delhi 2016)
Answer:
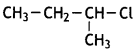 is more reactive.
is more reactive.
The SN1 reaction proceeds through the formation of a carbocation. The compound which forms a more stable carbocation will be more reactive.
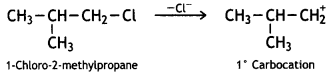
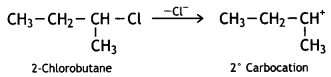
Since 2° carbocation is more stable than 1° carbocation, 2-Chlorobutane will be more reactive towards the SN1 reaction.
Question 18.
Write the structure of the product formed when chlorobenzene is treated with methyl chloride in the presence of sodium metal and dry ether. (CBSE Al 2018)
Answer:
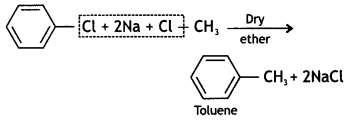
Question 19.
Which of the following two reactions is SN2 and why? (CBSE AI2016)
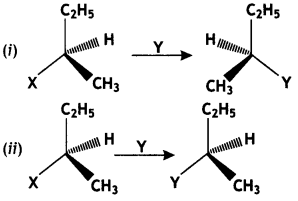
Answer:
Reaction (i) is SN2 because it proceeds by inversion of configuration.
Question 20.
Why is chloroform kept in dark coloured bottles? (CBSE AI 2019)
Answer:
Chloroform reacts with atmospheric oxygen in presence of light to produce an extremely poisonous gas called phosgene.
![]()
Chloroform is stored in dark coloured bottles so that it is not exposed to light and the formation of phosgene is prevented.
Question 21.
Write one stereochemical difference between SN1 and SN2 reactions. (CBSE Delhi 2019)
Answer:
SN1 reaction forms a racemic mixture while SN2 reaction results in inversion of configuration.
Question 22.
A solution of KOH hydrolyses CH3CHClCH2CH3 and CH3CH2CH2CH2Cl. Which one of these is more easily hydrolysed? (CBSE Delhi 2010)
Answer:
CH3CHClCH2CH3
Question 23.
Write the structure of 2, 4-dinitrochlorobenzene. (CBSE Delhi 2017)
Answer:
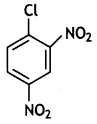
Question 24.
Write the structure of 1-Bromo-4- chlorobut-2-ene. (CBSE Delhi 2017)
Answer:
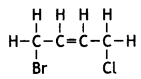
Question 25.
Write the structure of 3-Bromo-2- methylprop-1 -ene. (CBSE Delhi 2017)
Answer:
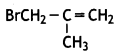
Question 26.
Write IUPAC name of the given compound: (CBSE Delhi 2019)
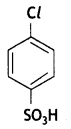
Answer:
4-Chlorobenzene sulphonic acid
Question 27.
Out of chlorobenzene and benzyl chloride, which one gets easily hydrolysed by aqueous NaOH and why? (CBSE Sample paper 2018)
Answer:
Benzyl chloride;
Due to resonance, stable benzyl carbocation is formed.
Haloalkanes and Haloarenes Important Extra Questions Short Answer Type
Question 1.
Which one of the following compounds will undergo hydrolysis at a faster rate by SN1 mechanism? Justify. (CBSE Sample Paper 2019)
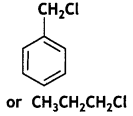
Answer:
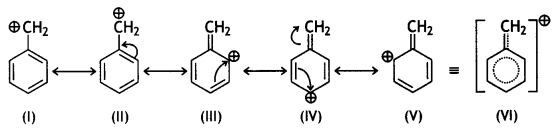
The following compound will undergo SN1 faster:
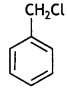
Greater the stability of the carbocation, greater will be its ease of formation from the corresponding halide and faster will be the rate of reaction. The benzylic carbocation formed gets stabilised through resonance.
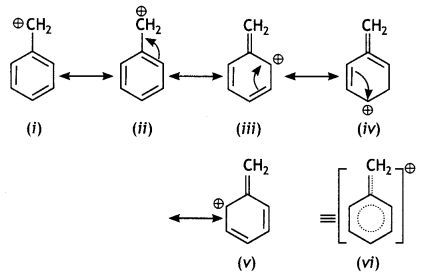
CH3CH2CH2Cl forms 1° carbocation, which is less stable than benzylic carbocation.
Question 2.
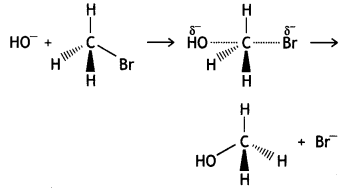
How would you differentiate between SN1 and SN2 mechanisms of substitution reactions? Give one example of each. (CBSE 2010)
Answer:
The SN1 reaction occurs in two steps and the reaction is of the first order.
The SN2 reaction occurs in one step and the reaction is of second order. These can also be distinguished as:
In SN1 reaction retention of the configuration takes place while in SN2 reaction inversion of the configuration takes place.
SN2 reaction:
SN1 reaction:
Question 3.
(i) State one use each of DDT and iodoform.
Answer:
DDT is used as an insecticide for killing mosquitoes and other insects. chloroform is used as an antiseptic.
(ii) Which compound in the following couples will react faster in SN2 displacement and why?
(a) I -Bromopentane or 2-bromopentane
Answer:
1-Bromopentane (1°) will react faster than 2-bromopentane (2°) by SN2 displacement because It is Less stericaLly hindered in the transition state.
(b) I-Bromo-2-methyl butane or 2-Bromo-2-methyl butane (CBSE Delhi 2010)
Answer:
1 -Bromo-2-methyl butane (1°) reacts faster than 2-bron,o-2-methyl butane (2°) because being primary, it will have Less steric hindrance.
Question 4.
Although chlorine Is an electron-withdrawing group, yet It Is ortho-, para-directing in electrophilic aromatic substitution reactions. Explain why Is It so? (CBSE 2012)
Answer:
Chlorine is an electron-withdrawing group, yet it is ortho-, para-directing in eLectrophiLic aromatic substitution reactions due to eLectron reLeasing resonance effect (+ R-effect).
As can be seen from the above resonating structures that -Ct increases electron density at ortho- and para-positions due to the + R-effect. Therefore, the attack of electrophile takes pLace at ortho- and paro- positions and not at meta- positions.
Question 5.
(i) Which alkyl halide from the following pair Is chiral and undergoes a faster SN2 reaction? (CBSE Delhi 2014)

Answer:
![]() is chiral
is chiral
(ii) Out of SN1 and SN2 which reaction occurs with
(a) Inversion of configuration
Answer:
SN2
(b) Racemisation
Answer:
SN1
Question 6.
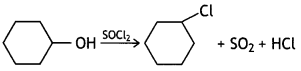
Draw the structure of major moon halo product In each of the following reactions: (CBSE Delhi 2014)
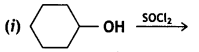
Answer:
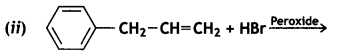
Answer:
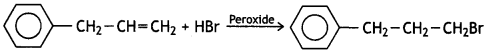
Question 7.
Propose the mechanism of the reaction taking place when
(i) (-) – 2 – Bromooctane reacts with sodium hydroxide to form (+) – octane-2-ol.
Answer:
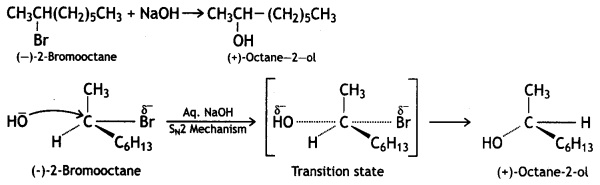
(ii) 2-Bromopentane is heated with (ale.) KOH to form alkenes. (CBSE Sample Paper 2011)
Answer:
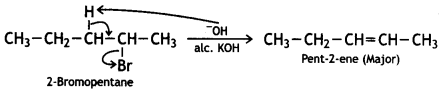
Question 8.
Out of ![]() and
and![]() which is more reactive towards SN1 reaction
which is more reactive towards SN1 reaction
Answer:
 is more reactive.
is more reactive.
The SN1 reaction proceeds through the formation of a carbocation. The compound which forms a more stable carbocation will be more reactive.
Since 2° carbocation is more stable than 1° carbocation, 2-Chlorobutane will be more reactive towards the SN1 reaction.
Haloalkanes and Haloarenes Important Extra Questions Long Answer Type
Question 1.
(i) Out of (CH3)3C-Br, and (CH3)3C-I, which one is more reactive towards SN1 and why?
Answer:
(CH3)2 C-I, because it can readily form stable carbocation because the C – l bond is weaker than the C – Br bond.
(ii) Write the product formed when p-nitro chlorobenzene is heated with aqueous NaOH at 443 K followed by acidification.
Answer:
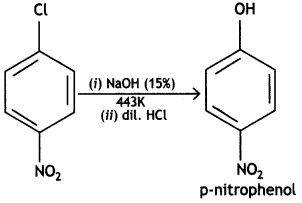
(iii) Why are dextro- and laevorotatory isomers of Butan-2-ol difficult to separate by fractional distillation? (CBSE Delhi 2019)
Answer:
Dextro- and laevorotatory isomers of butan-2-ol are enantiomers of each other and both have the same boiling point. Hence, these cannot be separated by fractional distillation.
Question 2.
Give reasons for the following:
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
Answer:
Ethyl iodide undergoes SN2 reaction faster than ethyl bromide because I- ion is a better leaving group than Br- ion.
(ii) (±) 2 – Butanol is optically inactive.
Answer:
(±) 2 – Butanol represents a racemic mixture of (+) 2-butanol and (-) 2-butanol which rotate the plane polarized light in different directions but to an equal extent. Therefore, the (±) compound is optically inactive.
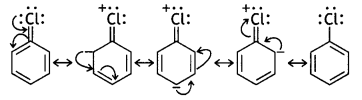
(iii) C—X bond length in halobenzene is smaller than C—X bond length in CH3 — X. (CBSE 2013)
Answer:
In halobenzene, there is delocalisation of electrons due to resonance. For example, chlorobenzene is considered to be a resonance hybrid of the following structures:
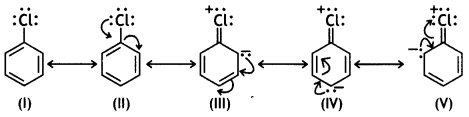
It is evident that the contribution of structures III, IV and V imparts a partial double bond character to the carbon-chlorine bond. Therefore, the C-X bond length in halobenzene is less than the C-X bond length in CH3-X, which has only a single C-X bond.
Question 3.
(i) Draw the structures of major moon halo products in each of the following reactions:
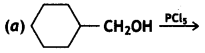
Answer:
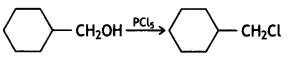
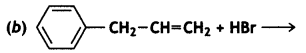
Answer:
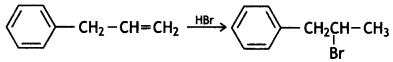
(ii) Which halogen compound in each of the following pairs will react faster in SN2 reaction:
(a) CH3Br or CH3I
Answer:
CH3I
(b) (CH3)3C – Cl or CH3 – Cl (CBSE 2014)
Answer:
CH3Cl
Question 4.
How would you convert the following:
(i) Prop-1-ene to 1-fluoropropane
(ii) Chlorobenzene to 2-chlorotoluene
(iii) Ethanol to propanenitrile
OR
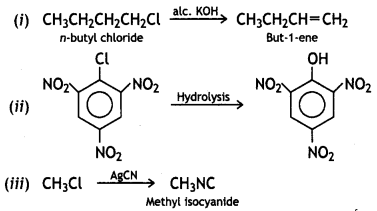
Write the main products when
(i) n-butyl chloride is treated with alcoholic KOH.
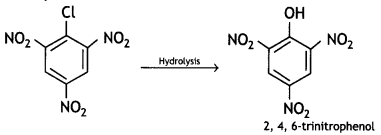
(ii) 2, 4, 6-trinitrochlorobenzene is subjected to hydrolysis.
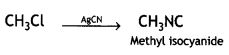
(iii) methyl chloride is treated with AgCN.
Answer:
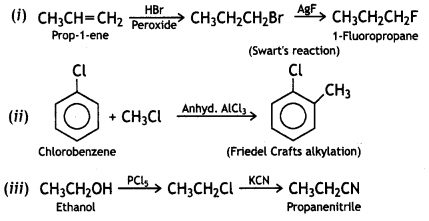
OR
Question 5.
Give reasons:
(i) C – Cl bond length in chlorobenzene is shorter than C — Cl bond length in CH3 — Cl.
Answer:
In chlorobenzene, there is delocalisation of electrons due to resonance.
It is considered to be a resonance hybrid of the following structures.
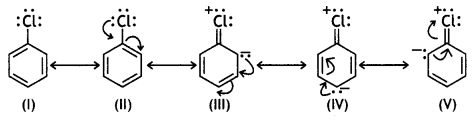
The contribution of structure III, IV and V imparts a partial double bond character to the C – Cl bond.
This is confirmed by X-ray analysis which shows that the C – Cl bond length in chlorobenzene is 1.69 whereas the C – Cl bond length in ethyl chloride is 1.82 A. There is no resonance in CH3Cl and hence no partial double bond character.
(ii) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Answer:
In chlorobenzene, the C of C-Cl bond is sp2-hybridised while the C of C-Cl bond in cyclohexyl chloride is sp3-hybridised.
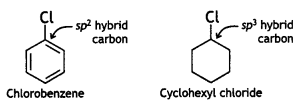
Therefore, the sp2-hybridised C of chlorobenzene has more s-character and hence more electronegative than the sp3-hybrid C of cyclohexyl chloride. As a result, the sp2 hybrid of the C-Cl bond in chlorobenzene has less tendency to release electrons to Cl than the sp3 hybrid carbon of cyclohexyl chloride. As a result, the C-Cl bond in chlorobenzene is less polar than in cyclohexyl chloride. Thus, chlorobenzene is less polar than cyclohexyl chloride.
(iii) SN1 reactions are accompanied by racemisation in optically active alkyl halides. (CBSE 2016)
Answer:
The SN1 reactions proceed through the formation of carbocations. In the case of optically active alkyl halides, the product formed is a racemic mixture. This is because the intermediate carbocation is planar species, therefore, the attack of the nucleophile, OFT ion can take place from both the faces (front and rear) with equal ease. As a result, a 50: 50 mixture of the two enantiomers (laevo and dextro) is formed. Thus, the product formed is a racemic mixture (±) which is optically inactive.
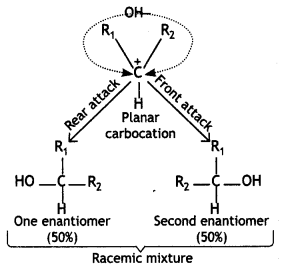
For example, hydrolysis of optically active 2-bromobutane results in the formation of a racemic mixture (±)-butan-2-ol.
Question 6.
The following compounds are given to you:
2-Bromopentane, 2-Bromo-2-methyl butane, 1-Bromopentane
(i) Write the compound which is most reactive towards the SN2 reaction.
Answer:
1-Bromopentane
(ii) Write the compound which is optically active.
Answer:
2-Bromopentane
(iii) Write the compound which is most reactive towards the SN2 elimination reaction.
Answer:
2-Bromo-2-methyl butane
Question 7.
Explain why
(i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride. (CBSE Delhi 2016)
Answer:
In chlorobenzene, the C of C-Cl bond is sp2-hybridised while the C of C-Cl bond in cyclohexyl chloride is sp3-hybridised.
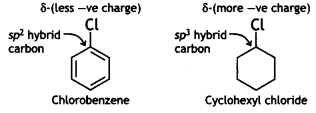
Therefore, the sp2-hybridised C of chlorobenzene has more s-character and hence more electronegative than the sp3-hybrid C of cyclohexyl chloride. As a result, the sp2 hybrid of the C-Cl bond in chlorobenzene has less tendency to release electrons to Cl than the sp3 hybrid carbon of cyclohexyl chloride. As a result, the C-Cl bond in chlorobenzene is less polar than in cyclohexyl chloride.
Thus, chlorobenzene is less polar than cyclohexyl chloride. In other words, the magnitude of negative charge (δ-) is less on the Cl atom of chlorobenzene than in cyclohexyl chloride. Further, due to the delocalisation of the lone pair of electrons of the Cl atom over the benzene ring due to resonance, the C-Cl bond in chlorobenzene acquires some double-bond character. On the other hand, the C-Cl bond in cyclohexyl chloride is a pure single bond.
Since dipole moment is a product of charge and distance, therefore, chlorobenzene has a lower dipole moment than cyclohexyl chloride due to the lower magnitude of charge (δ-) on Cl atom and small C-Cl distance.
(ii) alkyl halides, though polar, are immiscible with water,
Answer:
Alkyl halides are polar molecules and therefore, their molecules are held together by dipole-dipole forces. On the other hand, the molecules of H20 are held together by hydrogen bonds. When alkyl halides are added to water, the new forces of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide-alkyl halide molecules and water-water molecules. Hence, alkyl halides are immiscible (not soluble) in water.
(iii) Grignard reagents should be prepared under anhydrous conditions? (CBSE Sample Paper 2011)
Answer:
Grignard reagents are very reactive. They react with the moisture present in the apparatus or the starting materials (RX or Mg).
RMgX + HOH → R-H + Mg(OH)X
Therefore, Grignard reagents must be prepared in anhydrous conditions.
Question 8.
Which one of the following compounds will undergo a faster hydrolysis reaction by the SN1 mechanism? Justify your answer.
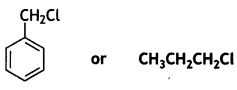
OR
A compound is formed by the substitution of two chlorine atoms for two hydrogen atoms in propane. Write the structures of the isomers possible. Give the IUPAC name of the isomer which can exhibit enantiomerism. (CBSE Sample paper 2018-19)
Answer:
C6 H5CH2Cl will undergo SN1 reaction faster.
The carbocation formed by C6 H5CH2Cl gets stabilised through resonance.
Greater the stability of carbocation, greater will be its ease of formation from the respective halide.
OR
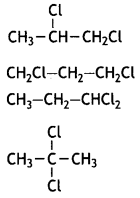
The following isomer will exhibit enantiomerism:
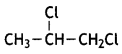
IUPAC name: 1,2-Dichloropropane.
Question 9.
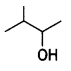
(i) Identify the chiral molecule in the following pair:
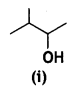 and
and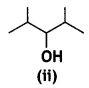
Answer:
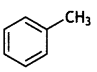
(ii) Write the structure of the product when chlorobenzene is treated with methyl chloride in the presence of sodium metal and dry ether.
Answer:
(iii) Write the structure of the alkene formed by dehydrohalogenation of 1-Bromo-1- methylcyclohexane with alcoholic KOH. (CBSE 2018)
Answer:
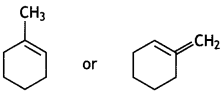
Question 10.
(a) Out of![]() and
and![]() which one is more reactive towards SN2 reaction and why?
which one is more reactive towards SN2 reaction and why?
Answer:
![]() because it is a primary halide and has less steric hindrance and therefore, undergoes SN2 action faster.
because it is a primary halide and has less steric hindrance and therefore, undergoes SN2 action faster.
(b) Out of![]() and
and![]() which one is more reactive towards nucleophilic substitution reaction and why?
which one is more reactive towards nucleophilic substitution reaction and why?
Answer:
![]() is more reactive because of the presence of electron-withdrawing – NO2 group.
is more reactive because of the presence of electron-withdrawing – NO2 group.
(c) Out of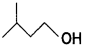 and
and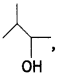 which one is optically active and why? (CBSE AI 2019)
which one is optically active and why? (CBSE AI 2019)
Answer:
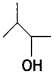 is optically active because of the presence of chiral carbon. OH
is optically active because of the presence of chiral carbon. OH
Question 11.
Write the products of the following reactions:
(i) CH3 CH2 -CH = CH2 + HCl →
Answer:
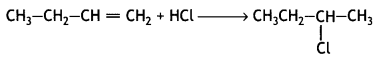
(ii) Me2CHCH2OH ![]()
Answer:
Me2CHCH2OH ![]() MeCHCH2Cl
MeCHCH2Cl
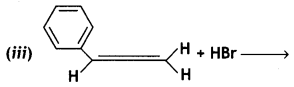
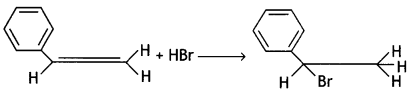
Answer:
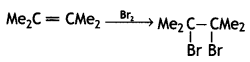
(iv) Me2C = CMe2 ![]()
Answer:
(v) 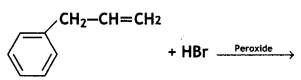 (CBSE AI 2016)
(CBSE AI 2016)
Answer:
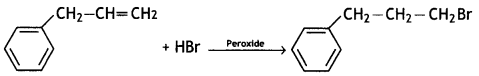
Question 12.
(i) In the following pairs of the halogen compounds, which would undergo SN2 faster?
Answer:
SN2 reaction proceeds through the formation of a transition state involving the bonding of carbon to five atoms or groups. The reactivity is decided by the stability of the transition state on the basis of steric hindrance. The reactivity follows the order: CH3 > 1° > 2° > 3° halide.
Therefore,
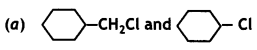
Answer:
![]() is primary alkyl halide and hence undergoes SN2 reaction faster.
is primary alkyl halide and hence undergoes SN2 reaction faster.
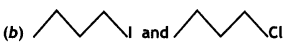
Answer:
![]() I will undergo SN2 reaction faster because iodine is a better leaving group due to its large size and hence it will be released at a faster rate in the presence of incoming nucleophile.
I will undergo SN2 reaction faster because iodine is a better leaving group due to its large size and hence it will be released at a faster rate in the presence of incoming nucleophile.
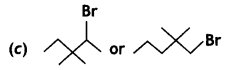
Answer:
![]() (1 -Bromo-2, 2-dimethylpentane) reacts faster because it is 1 ° alkyl halide.
(1 -Bromo-2, 2-dimethylpentane) reacts faster because it is 1 ° alkyl halide.
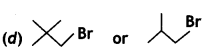 (CBSE AI 2009)
(CBSE AI 2009)
Answer:
![]() (2-Methyl-1-bromopropane) reacts faster because it has lesser steric hindrance in the transition state.
(2-Methyl-1-bromopropane) reacts faster because it has lesser steric hindrance in the transition state.
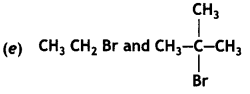
Answer:
CH3 CH2 Br would undergo SN2 reaction faster because of less steric hindrance.
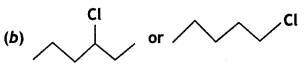
(ii) Which one of the following pairs undergoes SN1 substitution reaction faster and why?
Answer:
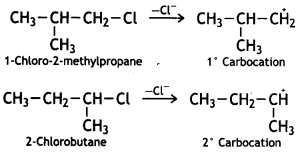
SN1 reaction occurs through the formation of a carbocation. Therefore, the greater the stability of the carbocation, the faster is the rate of an SN1 reaction. Since the stability of carbocation follows the order 3° > 2° > 1° > CH3, therefore,
Answer:
Out of ![]() or
or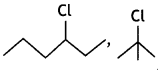 reacts faster because it invoLves the formation of 3° -carbocation
reacts faster because it invoLves the formation of 3° -carbocation![]() while
while 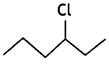 involves the formation of V -carbocation
involves the formation of V -carbocation![]()
 (CBSE AI 2009)
(CBSE AI 2009)
Answer:
Out of 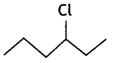 or
or 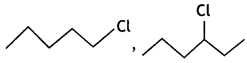 reacts faster because it forms 2° carbocation while
reacts faster because it forms 2° carbocation while ![]() involves 1° carbocation.
involves 1° carbocation.
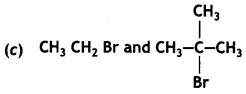 (CBSE AI 2015)
(CBSE AI 2015)
Answer:
 undergoes SN1 reaction faster. This is because the carbocation derived from CH3(CH3)3 C Br is 3° carbocation and is more stable than carbocation (1°) obtained from CH3 CH2 Br.
undergoes SN1 reaction faster. This is because the carbocation derived from CH3(CH3)3 C Br is 3° carbocation and is more stable than carbocation (1°) obtained from CH3 CH2 Br.
Question 13.
(i) How would you convert the following:
(a) Prop-1-ene to 1-fluoropropane
Answer:
![]()
(b) Chlorobenzene to 2-chlorotoluene
Answer:
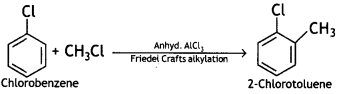
(ii) Write the main products when
(a) n-butyl chloride is treated with alcoholic KOH.
Answer:
![]()
(b) 2, 4, 6-trinitrochlorobenzene is subjected to hydrolysis.
Answer:
(c) methyl chloride is treated with AgCN. (CBSE AI2015)
Answer:
Question 14.
Compound ‘A’ with molecular formula C4H9Br is treated with aq. KOH solution. The rate of this reaction depends upon the concentration of the compound ‘A’ only. When another optically active isomer ‘B’ of this compound was treated with aq. KOH solution, the rate of reaction was found to be dependent on the concentration of compound and KOH both.
(i) Write down the structural formula of both compounds ‘A’ and ‘B’.
Answer:
The molecular formulae of isomers of C4H9Br are CH3
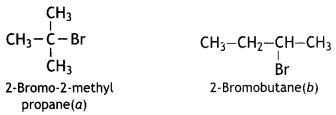
Since the rate of reaction of the compound ‘A’ (C4H9Br) with aqueous KOH depends upon the concentration of compound ‘A’ only, therefore, the reaction occurs by SN1 mechanism and compound ‘A’ is tertiary bromide, i.e. 2-Bromo-2-methylpropane.
(CH3)3CBr + KOH(aq) → (CH3 )3COH + KBr rate = k[(CH3)3CBr]
(ii) Out of these two compounds, which one will be converted to the product with an inverted configuration. (NCERT Exemplar)
Answer:
Since compound ‘B’ is optically active and is an isomer of compound ‘A’ (C4H9Br), therefore, compound ‘B’ must be 2-bromobutane. Since the rate of reaction of compound ‘B’ with aqueous KOH depends upon the concentration of compound ‘B’ and KOH, therefore, the reaction occurs by SN2 mechanism and the product of hydrolysis will have inverted configuration.
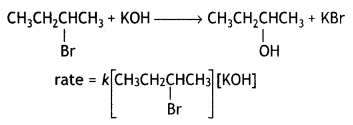
Compound ‘B’ will be converted with an inverted configuration.
Question 15.
(i) Draw other resonance structures related to the following structure and find out whether the functional group present in the molecule is ortho, para directing or meta directing.
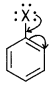
Answer:
It is clear from resonating structures that there is an increase in electron density at ortho and para positions. Therefore, the functional group ‘X’ is ortho and para directing.
(ii) Classify the following compounds as primary, secondary and tertiary halides.
(a) 1-Bromobut-2-ene
Answer:
Primary
(b) 4-Bromopent-2-ene
Answer:
secondary
(c) 2-Bromo-2-methylpropane (NCERT Exemplar)
Answer:
tertiary
Question 16.
tert-Butylbromide reacts with aq. NaOH by SN1 mechanism while n-butyl bromide reacts by an SN2 mechanism. Why?
Answer:
In general, the SN1 reaction proceeds through the formation of a carbocation. The tert-butyl bromide readily loses Br– ion to form stable 3° carbocation.
Therefore, it reacts with aqueous KOH by SN1 mechanism as:
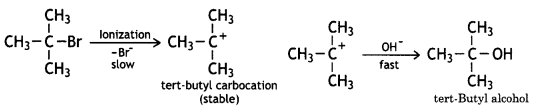
On the other hand, n-butyl bromide does not undergo ionisation to form n-butyl carbocation (1°) because it is not stable. Therefore, it prefers to undergo reaction by an SN2 mechanism, which occurs in one step through a transition state involving the nucleophilic attack of OH– ion from the backside with simultaneous expulsion of Br– ion from the front side.
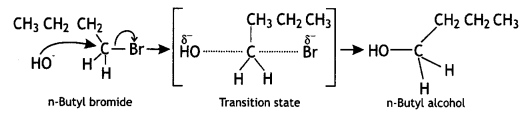
SN1 mechanism follows the reactivity order as 3° > 2° > 1° while the SN2 mechanism follows the reactivity order as 1° >2° >3°
Therefore, tert-butyl bromide (3°) reacts by SN1 mechanism while n-butyl bromide (1°) reacts by the SN2 mechanism.