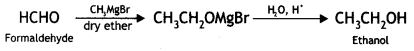Here we are providing Class 12 Chemistry Important Extra Questions and Answers Chapter 11 Alcohols, Phenols and Ethers. Class 12 Chemistry Important Questions are the best resource for students which helps in Class 12 board exams.
Class 12 Chemistry Chapter 11 Important Extra Questions Alcohols, Phenols and Ethers
Alcohols, Phenols and Ethers Important Extra Questions Very Short Answer Type
Question 1.
Arrange the following in increasing order of their boiling point:
CH3CH2OH, CH3CHO, CH3—O—CH3 (CBSE AI 2019)
Answer:
CH3—O—CH3 < CH3CHO < CH3CH2OH
Question 2.
Arrange the following in increasing order of their acidic character:
Ethanol, Phenol, Water (CBSE AI 2019)
Answer:
Ethanol <Water < Phenol
Question 3.
How will you convert ethanol to ethene? (CBSE 2011)
Answer:
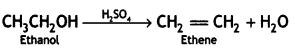
Question 4.
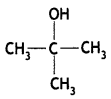
Draw the structural formula of the 2-methylpropan-2-ol molecule. (CBSE Delhi 2012)
Answer:
Question 5.
Which of the following isomers is more volatile: (CBSE Delhi 2014)
o-nitrophenol or p-nitrophenol?
Answer:
o-nitrophenol
Question 6.
Write the IUPAC name of the given compound: (CBSE 2015)
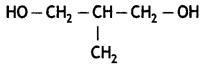
Answer:
2-Methylpropane-1, 3-diol
Question 7.
Write the IUPAC name of the given compound: (CBSE Delhi 2015)
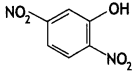
Answer:
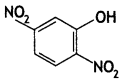 2, 5- Dinitrophenol
2, 5- Dinitrophenol
Question 8.
Arrange the following in increasing order of their acidic character: Benzoic acid, Phenol, Cresol (CBSE Al 2019)
Answer:
Cresol < Phenol < Benzoic acid
Question 9.
Rearrange the following compounds in the increasing order of their boiling points: (CBSE Al 2013)
CH3-CHO, CH3-CH2-OH, CH3-CH2-CH3
Answer:
CH3CH2CH3 < CH3CHO < CH3CH2OH
Question 10.
An alkoxide is a stronger base than a hydroxide ion. Justify. (CBSE Sample Paper 2012)
Answer:
Due to the presence of electron-donating alkyl group, there is high electron density in alkoxide ion as compared to hydroxide ion. Therefore, the alkoxide ion is more basic than the hydroxide ion.
Question 11.
Why is (±) butan-2-ol optically inactive? (CBSE Delhi 2013, CBSE AI 2013)
Answer:
(±)-Butan-2-ol represents a racemic mixture of (+)-butan-2-ol and (-)-2-butanol which rotate the plane polarized light in different directions but to an equal extent. Therefore, (±) compound is optically inactive.
Question 12.
Write the IUPAC name of the following compound: (CBSE Al 2017)
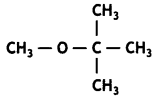
Answer:
2-Methoxy-2-methylpropane
Question 13.
Write the IUPAC name of the following compound: (CBSE AI 2017)
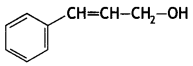
Answer:
3-Phenylprop-2-en-1-ol
Question 14.
Write the IUPAC name of the following: (CBSEAI 2018)
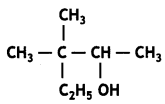
Answer:
3, 3-Dimethylpentan-2-ol
Alcohols, Phenols and Ethers Important Extra Questions Short Answer Type
Question 1.
Predict the major product obtained when t-butyl bromide reacts with sodium methoxide. Also, give its IUPAC name.
Or
(a) Show the chemical reaction with bond movements and arrows for the nucleophilic attack of water on carbocation in acid catalysed hydration of alkenes.
(b) Give IUPAC name for the following: (CBSE 2019C)
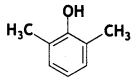
Answer:
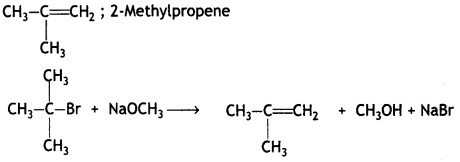
OR
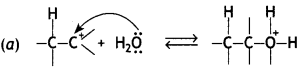
Mechanism: hydration of alkenes
The mechanism of the reaction involves the following steps:
Step 1. Alkene gets protonated to form a carbocation by the electrophilic attack of H3O+:
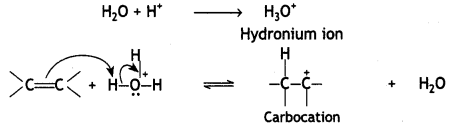
Step 2. Nucleophile (H20) attacks the carbocation forming protonated alcohol.
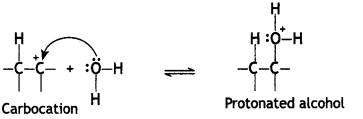
Step 3. Loss of H+ from oxygen (deprotonation) to form alcohol
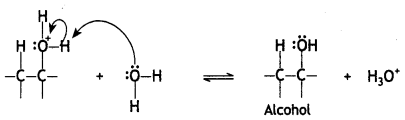
(b) 2,6-dimethylphenol
Question 2.
How are the following conversions carried out?
(i) Propene to propan-2-ol
Answer:
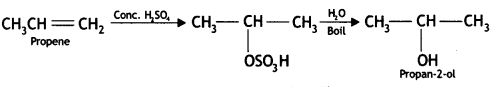
(ii) Ethyl magnesium chloride to propan-1-ol (CBSE Delhi 2010)
Answer:
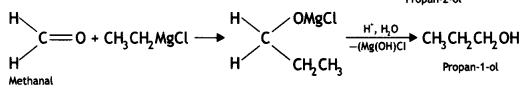
Question 3.
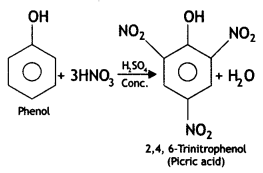
How would you obtain:
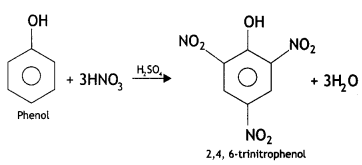
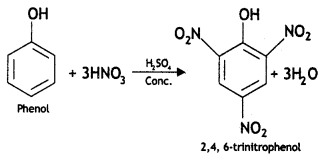
(i) Picric add (2, 4, 6-trlnitrophenol) from phenol.
Answer:
(ii) 2-Methyl propene from 2-methyl propanol? (CBSE Delhi 2011)
Answer:
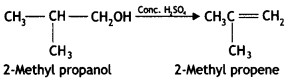
Question 4.
Write the chemical equations involved in the following reactions:
(i) Kolbe’s reaction
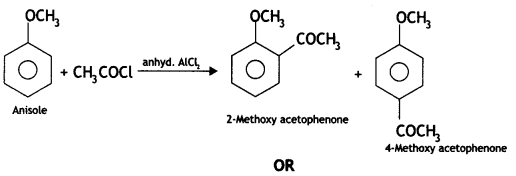
(ii) Friedel-Crafts acetylation of anisole (CBSE Delhi 2016)
OR
How do you convert:
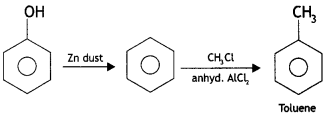
(i) Phenol to toluene
(ii) Formaldehyde to ethanol?
Answer:
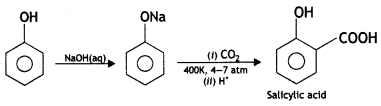
(i) Kolbe’s reaction. Sodium phenoxide reacts with CO2 under pressure (6-7 atm) at 400 K to form sodium salicylate which upon acidification with HCL gives salicylic acid.
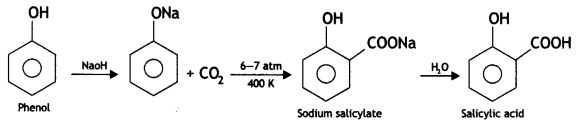
(ii) Friedel-Crafts acetylation of anisole. On reacting anisole with acetyl chloride in the presence of anhyd. AlCl3, 2-methoxy acetophenone and 4-methoxy acetophenone are formed.
(i) Phenol to toluene
(ii) Formaldehyde to ethanol

Question 5.
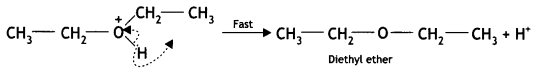
Explain the mechanism of the following reaction: (CBSE Delhi 2013, 2016)
![]()
Answer:
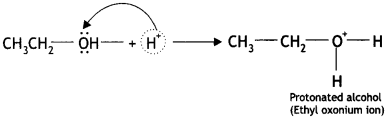
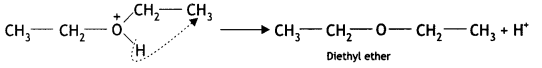
The mechanism for the formation of diethyl ether from ethanol at 413 K is given below:
(i) Ethyl alcohol gets protonated in the presence of H+.
(ii) Nucleophilic attack by another alcohol (unprotonated) molecule occurs on the protonated alcohol with the elimination of a molecule of water.
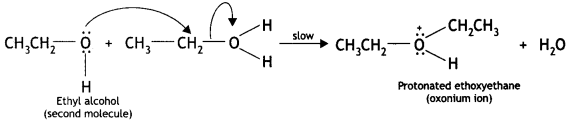
(iii) Oxonium ion [oses a proton to form an ether.
Question 6.
How will you convert:
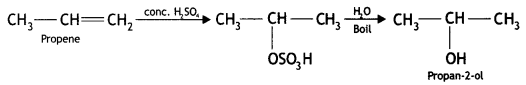
(i) Propene to Propan-2-ol?
Answer:
(ii) Phenol to 2, 4, 6 – trinitrophenol? (CBSE Delhi 2013)
Answer:
Question 7.
How will you convert:
(i) Propene to propan-1 -ol?
Answer:
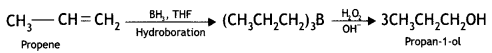
(ii)Ethanal to propan-2-ol?
Answer:
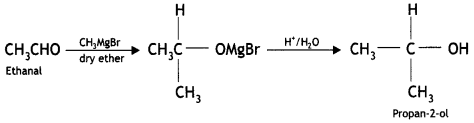
Question 8.
Give chemical tests to distinguish between (CBSE Sample Paper 2011)
(i) Isopropyl alcohol and n-propyl alcohol
Answer:
Isopropyl alcohol gives the iodoform test. On heating with NaOH/l2 or NaOl, isopropyl alcohol forms a yellow precipitate of iodoform (CHI3).
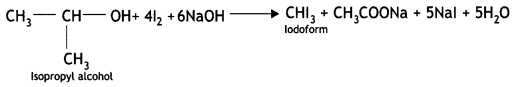
(ii) Phenol and alcohol.
Answer:
Phenol reacts with neutral FeCl3 solution to give red-violet complex, whereas alcohol does not give this test.
Question 9.
(i) Arrange the following compounds in the increasing order of their acid strength:
p-cresol, p-nitrophenol, phenol
Answer:
p-cresol < phenol < p-nitrophenol
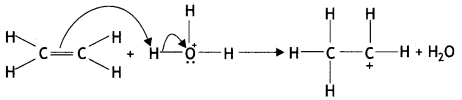
(ii) Write the mechanism (using curved arrow notation) of the following reaction:
(a) CH2 = CH2 ![]() CH3-CH2 + H2O
CH3-CH2 + H2O
OR
Write the structures of the products when butan-2-ol reacts with the following: (CBSE AI 2017)
(i) CrO3
(ii) SOCl2
Answer:
Or
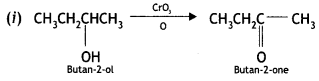
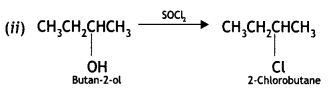
Question 10.
Alcohols are easily protonated in comparison to phenols. Explain. (CBSE AI 2016)
Answer:
In alcohols, the electron-releasing inductive effect (+ I effect) of the alkyl group attached to the carbon having the -OH group increases the electron density on the oxygen atom. Therefore, alcohols are easily protonated.
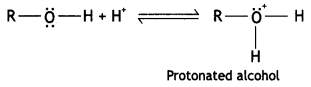
On the other hand, in the case of phenol, the oxygen atom acquires a partial positive charge due to resonance.
Thus, it is not protonated.
Question 11.
Explain why ortho nitrophenol is more acidic than ortho methoxy phenol. (CBSE AI 2015)
Answer:
This is because -NO2 (nitro group) is an electron-withdrawing group and will increase the +ve charge on oxygen to make it more acidic. On the other hand, the -OCH3 group is an electron-releasing group and will decrease +ve charge on oxygen making it less acidic as the O-H bond will not break easily.
Question 12.
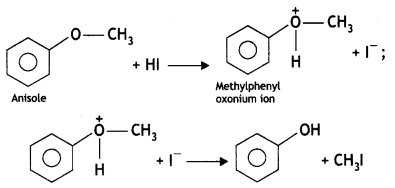
Anisole on reaction with HI gives phenol and CH3I as main products and not iodobenzene and CH3OH. Why? (CBSE AI 2016)
Answer:
It this reaction protonated anisole, i.e. methyl phenyl oxonium ion, is first formed and then the halide ion attacks this protonated anisole. Due to steric hindrance of the bulky phenyl group, the attack preferably occurs to the alkyl group forming methyl iodide and phenol.
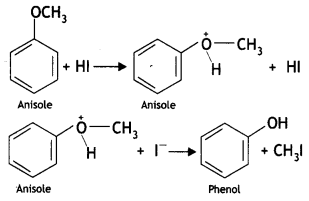
Question 13.
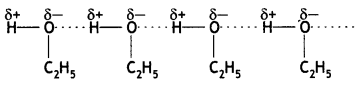
The boiling points of ethers are lower than their corresponding isomeric alcohols. Explain. (CBSE AI 2012)
Answer:
Ethers have low polarity and therefore, do not show any association by intermolecular hydrogen bonding. On the other hand, their isomeric alcohols have strong intermolecular hydrogen bonding and therefore, their boiling points are high.
Question 14.
Butan-1-ol has a higher boiling point than diethyl ether. Give reason.
Answer:
Butan-1-ol has intermolecular hydrogen bonding between their molecules. Therefore, it exists as associated molecules and a large amount of energy is required to break these bonds and hence its boiling point is high. But diethyl ether does not show any association by intermolecular hydrogen bonding. Hence, its boiling point is low.
Question 15.
Write the mechanism of acid dehydration of ethanol to yield ethene. (CBSE Sample Paper 2018)
Answer:
The reaction is believed to occur as follows:
(i) Formation of protonated alcohol: Alcohol combines with a proton to form a protonated alcohol.
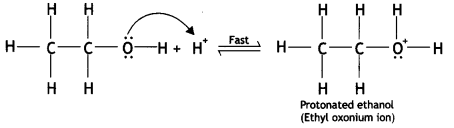
(ii) Formation of the carbocation: The protonated alcohol loses a water molecule to form a carbocation.
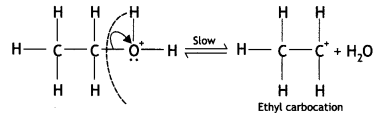
(iii) Elimination of a proton to form alkene: The carbocation then eliminates a proton and undergoes rearrangement of electrons to form the alkene.
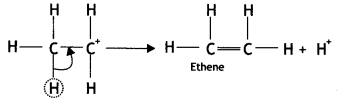
The acid used in step (i) is released in step (iii). To drive the equilibrium reaction in the forward direction, ethene is removed as soon as it is formed.
Alcohols, Phenols and Ethers Important Extra Questions Long Answer Type
Question 1.
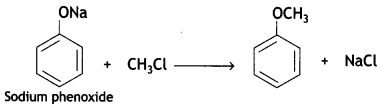
What happens when
(a) Sodium phenoxide is treated with CH3Cl?
Answer:
(b) CH2 = CH – CH2 – OH is oxidised by PCC?
Answer:
![]()
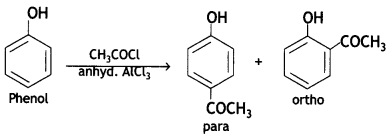
(c) Phenol is treated with CH3COCI/anhydrous AlCl3?
Write chemical equations in support of your answer.
Answer:
Question 2.
(a) How will you convert the following:
(i) Phenol to benzoquinone
Answer:
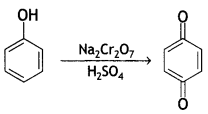
(ii) Propanone to 2-methyl propane-2-ol
Answer:
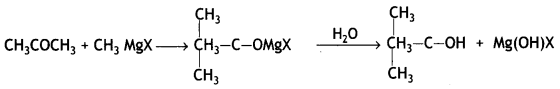
(b) Why does propanol have a higher boiling point than that butane? (CBSE 2019C)
Answer:
The molecules of propanol are held together by intermolecular hydrogen bonding while butane molecules have only weak van der Waals forces of attraction. Since hydrogen bonds are stronger than van der Waals forces, therefore, propanol has a higher boiling point than butane.
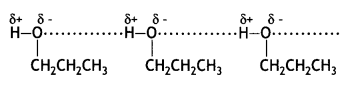
Question 3.
Identify the product formed when propan-1 -ol is treated with a cone. H2S04 at 413 K. Write the mechanism involved for the above reaction. (CBSE Sample Paper 2019)
Answer:
(a) 1-Propoxypropane is formed.
Mechanism involved:
Step 1: Formation of protonated alcohol. Propanol gets protonated in the presence of H+.
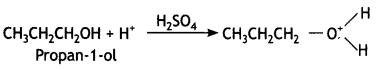
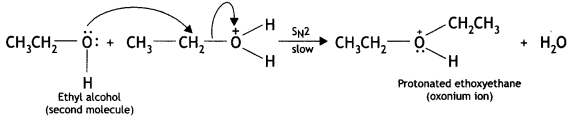
Step 2: Nucleophilic attack. Due to the presence of a +ve charge on the oxygen atom, the carbon of the CH2 part becomes electron deficient. As a result, a nucleophilic attack by another alcohol molecule (unprotonated) occurs on the protonated alcohol with the elimination of a molecule of water.
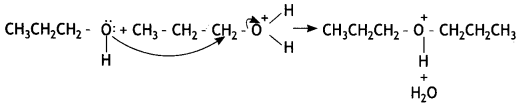
Step 3: Deprotonation. Oxonium ion loses a proton to form an ether.
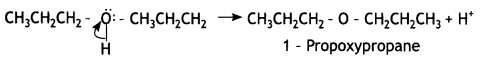
Question 4.
Write the equations involved in the following reactions: (CBSE 2013, 2014)
(i) Reimer-Tiemann reaction
Answer:
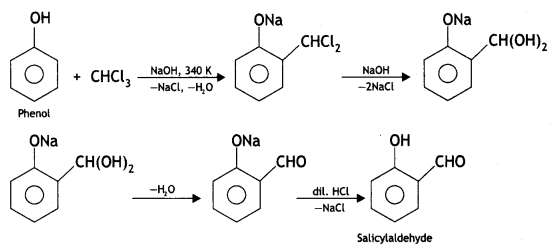
Reimer-Tiemann reaction: When phenol is refluxed with chloroform in the presence of aqueous caustic alkali at 340 K, an aldehydic group (CHO) gets introduced in the ring at a position ortho to the phenolic group. Ortho hydroxy benzaldehyde or salicylaldehyde is formed as the product of the reaction.
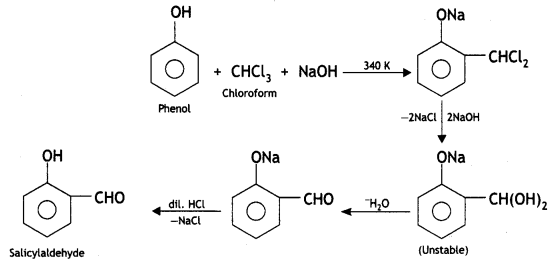
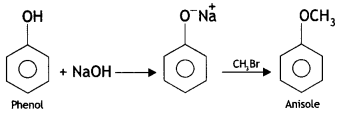
(ii) Williamson’s ether synthesis
Answer:
Williamson’s ether synthesis. This is used to prepare symmetrical and unsymmetrical ethers by treating alkyl halide with either sodium alkoxide or sodium phenoxide.
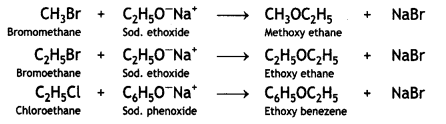
Aryl halides cannot be used for the preparation of alkyl-aryl ethers because of their low reactivity.
Question 5.
Draw the structure and name the product formed if the following alcohols are oxidised. Assume that an excess of the oxidising agent is used. (CBSE Delhi 2012)
(i) CH3CH2CH2CH2OH
Answer:
![]()
(ii) 2-butenol
Answer:

(iii) 2-methyl-1-propanol
Answer:
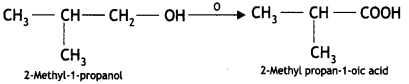
Question 6.
Write the main product(s) in each of the following reactions: (CBSE Delhi 2016)
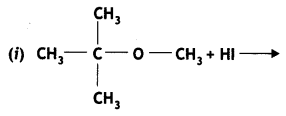
Answer:
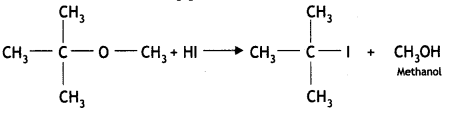
![]()
Answer:
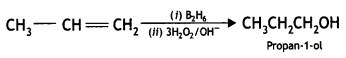
![]()
Answer:
Question 7.
Give reasons for the following: (CBSE Delhi 2016, Outside Delhi)
(i) Protonation of phenols Is difficult whereas ethanol easily undergoes protonation.
Answer:
In phenols, the oxygen atom acquires a partial positive charge due to resonance and therefore, it is not easily protonated. On the other hand, in ethanol, the alkyl group is an electron-releasing group and increases the electron density on 0 atoms. Therefore, ethanol is easily protonated.
(ii) Bolting point of ethanol is higher than that of dimethyl ether.
Answer:
The molecules of ethanol are held together by intermolecular hydrogen bonding while dimethyl molecules have only weak van der Waals forces of attractions. Since hydrogen bonds are stronger than van der Waals forces, ethanol has a higher boiling point than dimethyl ether.
(iii) Anisole on reaction with Hl gives phenol and CH3-I as main products and not iodobenzene and CH3OH.
Answer:
This is because, during the reaction, the attack of halide ion occurs to the protonated anisole, i.e. methyl phenyl oxonium ion, which is formed during the protonation of anisole. Due to steric hindrance of the bulky phenyl group, the attack preferably occurs to the alkyl group forming methyl iodide and phenol.
Question 8.
How do you convert the following: (CBSE Delhi 2015, Delhi)
(i) Phenol to anisole
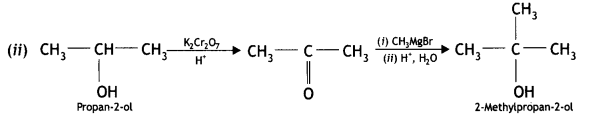
(ii) Propan-2-ol to 2-methylpropan-2-ol
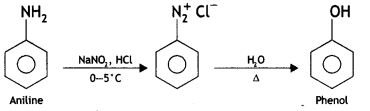
(iii) Aniline to phenol?
OR
(i) Write the mechanism of the following reaction:
2CH3CH2OH ![]() CH3CH2—O—CH2CH3
CH3CH2—O—CH2CH3
(ii) Write the equation involved In the acetylation of salicylic acid.
Answer:
(i) Phenol to anisole
(iii) Aniline to phenol
Or
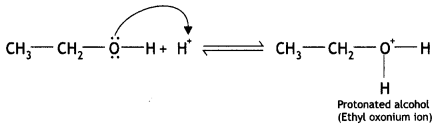
(i) The mechanism for the formation of ether from ethanol at 413 K is a nucleophilic bimolecular reaction as given below:
(a) Ethyl alcohol gets protonated in the presence of H+
(b) Due to the presence of a +ve charge on the oxygen atom, the carbon of CH2 part of CH3CH2 becomes electron deficient. As a result, nucleophilic attack by another alcohol molecule (unprotonated) occurs on the protonated alcohol with the elimination of a
(c) Oxonium ion loses a proton to form an ether.
Question 9.
Give reasons for the following: (CBSE 2015, Outside Delhi)
(i) o-nitrophenol is more acidic than o-methoxyphenyl.
Answer:
This is because —NO2 (nitro group) is an electron-withdrawing group and will increase the +ve charge on oxygen to make it more acidic. On the other hand, the -OCH3 group is an electron¬releasing group and will decrease +ve charge on oxygen making it less acidic as O-H bond will not break easily.
(ii) Butan-1-oi has a higher boiling point than diethyl ether.
Answer:
Butan-1 -ol has intermolecular hydrogen bonding between their molecules. Therefore, it exists as associated molecules and large amount of energy is required to break these bonds and hence, its boiling point is high. But diethyl ether does not show any association by intermolecular hydrogen bonding. Hence, its boiling point is low.
(iii) (CH3)3C – O – CH3 on reaction with HI gives (CH3)3C – I and CH3 – OH as the main products and not (CH3)3C – OH and CH3 – I.
Answer:
The reaction:
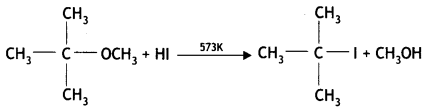
gives (CH3)3C – I and CH3OH as the main products and not (CH3)3COH and CH3I. This is because the reaction occurs by the SN1 mechanism and the formation of products is governed by the stability of carbocation formed from the cleavage of the C-0 bond in the protonated ether. Since tert. butyl carbocation, (CH3)3C+ is more stable than methyl carbocation, CH3, the cleavage of C-0 gives a more stable carbocation, [(CH3)3C]+ and methanol. Then, iodide ion, I– attacks this tert. butyl carbocation to form tert. butyl iodide.
Question 10.
(i) Write the mechanism of the following reaction: (CBSE 2015, Outside Delhi)
![]()
Answer:
HBr → H+ + Br–
(a) H+ attacks oxygen of O-H to form protonated alcohol
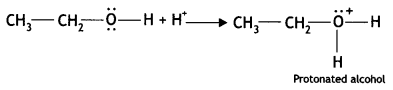
(b) Protonated alcohol Loses a molecule of water to form a carbocation.
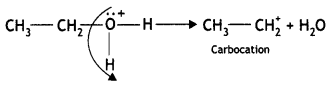
(C) Br attacks the carbocation to form bromoatkane
CH3CH2+ + Br– → CH3CH2Br (Bromoethane)
(ii) Write the equation involved in the Reimer-Tiemann reaction.
Question 11.
(A), (B) and (C) are three non-cyclic functional isomers of a carbonyl compound with molecular formula C4H80. Isomers (A) and (C) give positive Tollens’ test whereas isomer (B) does not give Tollens’ test but gives positive Iodoform test. Isomers (A) and (B) on reduction with Zn(Hg)/conc. HCl, give the same product (D).
(i) Write the structures of (A), (B), (C) and (D).
Answer:
A = CH3CH2CH2CHO
B = CH3COCH2CH3
C = (CH3)2CHCHO
D = CH3CH2CH2CH3
(ii) Out of (A), (B) and (C) isomers, which one is least reactive towards the addition of HCN? (CBSE 2018 C)
Answer:
B
Question 12.
(a) How will you convert the following:
(i) Phenol to benzene
Answer:
(ii) Propene to propanol
Answer:
![]()
(b) Why is ortho-nitrophenol more acidic than ortho-methoxy phenol? (CBSE 2018C) OH
Answer:
Due to the strong -R and -I effect of the -NO2 group, the electron density in the O-H bond decreases and hence the loss of a proton becomes easy.

Moreover, the o-nitrophenoxide formed after the Loss of a proton is stabilised by resonance.
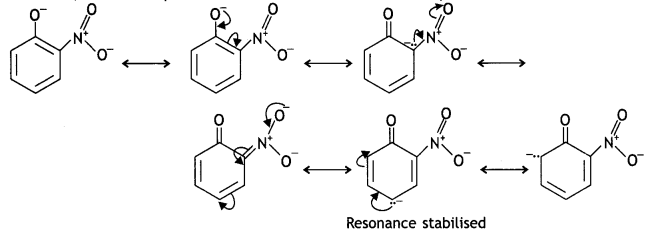
o-nitrophenoxide ion is stabilised by resonance and hence o-nitrophenol is a stronger acid. On the other hand, due to the +R effect of the -OCH3 group, the electron density in the O-H bond increases and this makes the loss of proton difficult.
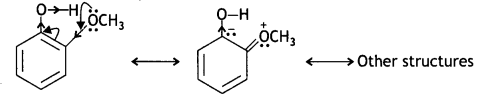
Furthermore, after the Loss of proton o-methoxyphenoxide ion left is destabilised by resonance.
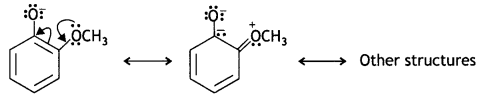
The two negative charges repel each other and therefore, destabilise the o-methoxyphenoxide ion. Thus, o-nitrophenol is more acidic than o-methoxyphenyl.
Question 13.
(a) How will you convert the following:
(i) Ethanal to propan-2-ol
Answer:
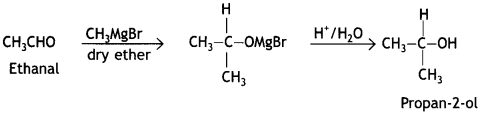
(ii) Phenol to 2-hydroxy acetophenone
Answer:
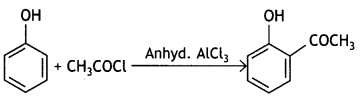
(b) Why is phenol more acidic than ethanol? (CBSE 2019C)
Answer:
It is because phenoxide ion is more stable than ethoxide ion. Phenols are more acidic than alcohols. The greater acidic character of phenoLs as compared to alcohols can be explained on the basis of resonance. Phenol is a resonance hybrid of the following structures:
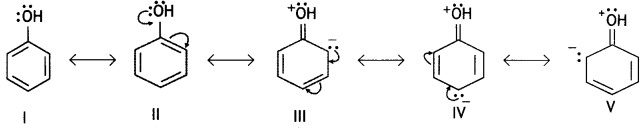
It is clear that three structures of phenol (III, IV and V) have a +ve charge on the oxygen of the —OH group. This oxygen attracts the electron pair of O—H bond strongly towards itself, weakens the O—H bond and, therefore, facilitates the release of W. Similarly, the phenoxide ion is resonance stabilised as follows:
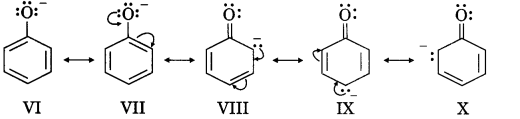
Thus, we observe that both phenol and phenoxide ion are stabilised by resonance. Now if we carefully observe the resonance structures, we observe that phenoxide ion is more resonance stabilised than phenol. In phenol three contributing structures (III, IV and V) have both positive and negative charges and therefore, these will be unstable. These structures will require energy to separate the charge and therefore, will be unstable.
On the other hand, there is no structure in phenoxide ion which requires charge separation. Thus, the resonance hybrid of phenol is less stable than phenoxide ion and the reaction is very much in favour of the phenoxide ion. Therefore, the phenol is more acidic.
On the other hand, in the case of alcohols, neither the alcohol nor the alkoxide ion is stabilised by resonance.

Thus, phenols are more acidic than alcohols.
Question 13.
(a) How do you convert the following?
(i) Phenol to Anisole
(ii) Ethanol to Propan-2-ol
(b) Write the mechanism of the following reaction:
![]()
(c) Why phenol undergoes electrophilic substitution more easily than benzene?
OR
(a) Account for the following:
(i) o-Nitrophenol is more steam volatile than p-nitrophenol.
(ii) Tert-butyl chloride on heating with sodium methoxide gives 2-methylpropene instead of tert-butyl methyl ether.
(b) Write the reaction involved in the following:
(i) Reimer-Tiemann reaction
(ii) Friedel-Crafts Alkylation of Phenol
(c) Give a simple chemical test to distinguish between Ethanol and Phenol. (CBSE Delhi 2019)
Answer:
(i) 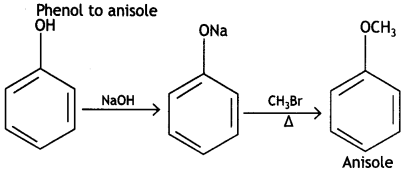
(ii) Ethanol to Propan-2-ol
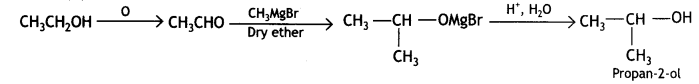
(b) Mechanism CH3
(i) Ethanol combines with a proton to form protonated alcohol.
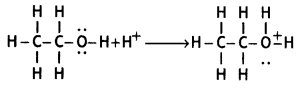
(ii) Protonated alcohol loses a water molecule to form a carbocation.
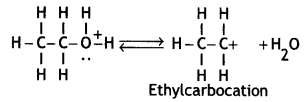
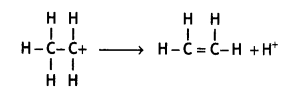
(iii) Carbocation eliminates a proton and undergoes rearrangement of electrons to form an alkene.
(c) In phenol, the -OH group is an activating group and therefore, electrophilic substitution reaction undergoes more easily in phenol than benzene.
OR
(a) (i) o-Nitrophenol is more steam volatile than p-nitrophenol because of chelation due to intramolecular hydrogen bonding.
(ii) Tert-Butyl chloride reacts with sodium methoxide to give 2-methylpropene.
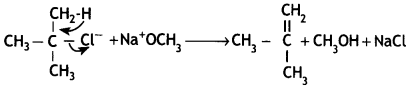
This is because alkoxides are not only nucleophiles but strong bases as well. They react with alkyl halides leading to an elimination reaction.
(b) Reimer-liemann reaction
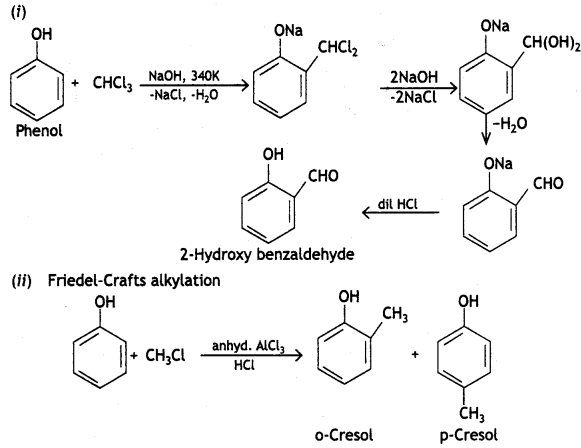
(C) Phenols react with neutral Fecl3 to give violet colour, while ethanol does not give any colour.
Question 14.
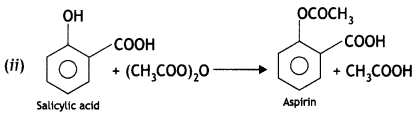
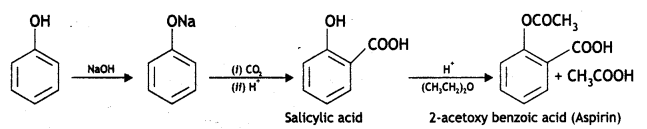
(i) Write steps to carry out the conversion of phenol to aspirin.
Answer:
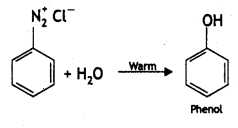
(ii) What happens when benzene diazonium chloride Is heated with water?
Answer:
(iii) Write the structures of the Isomers of alcohols with molecular formula C4H10O. Which of these exhibits optical activity?
Answer:
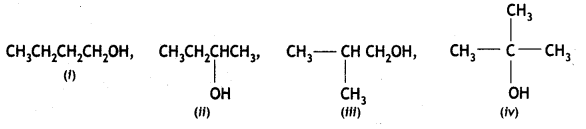
(ii) is optically active.
Question 15.
How are the following conversions carried out?
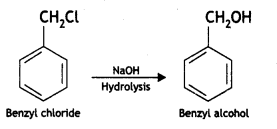
(i) Benzyl chloride to benzyl alcohol
Answer:
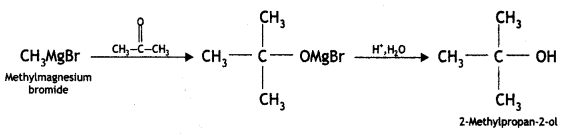
(ii) Methyl magnesium bromide to 2-methyl propane-2-ol
Answer:
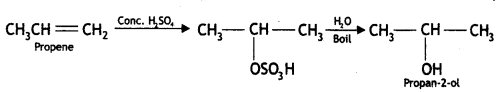
(iii) Propene to propan-2-ol
Answer:
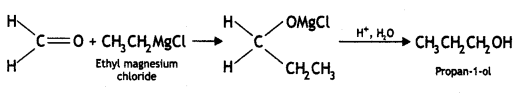
(iv) Ethylmagnesium chloride to propan-1-ol. (CBSE Delhi 2010, 2013)
Answer:
Question 16.
How will you convert:
(i) Phenol to 2, 4, 6-trlnftrophenot
Answer:
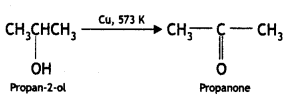
(ii) Propan-2-ol to propanone
Answer:
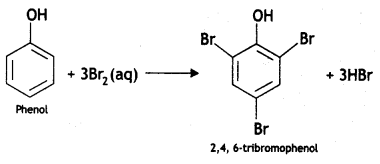
(iii) Phenol to 2, 4, 6-trlbromophenol
Answer:
(iv) Propene to propan-1-ol
Answer:
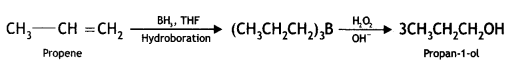
(v) Ethanal to propan-2-ol? (CBSE DelhI 2013)
Answer:
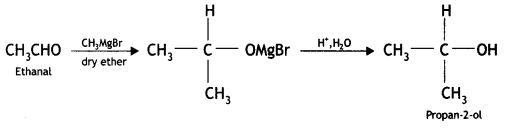
Question 17.
How will you convert
(i) Propan-2-ol to 2-methylpropan-2-ol (CBSE Delhi 2015)
Answer:
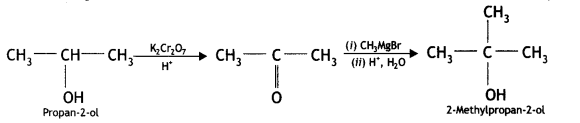
(ii) Aniline to phenol (CBSE Delhi 2015)
Answer:
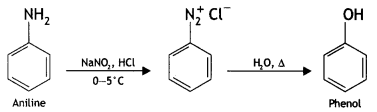
(iii) Ethanol to propane nitrile (CBSE AI 2015)
Answer:
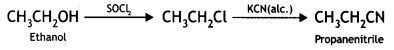
(iv) Phenol to toluene (CBSE AI 2016)
Answer:
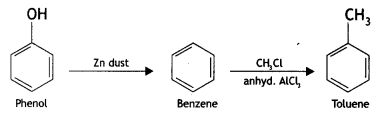
(v) Formaldehyde to ethanol? (CBSE AI 2016)
Answer: