Here we are providing Class 12 Chemistry Important Extra Questions and Answers Chapter 14 Biomolecules. Class 12 Chemistry Important Questions are the best resource for students which helps in Class 12 board exams.
Class 12 Chemistry Chapter 14 Important Extra Questions Biomolecules
Biomolecules Important Extra Questions Very Short Answer Type
Question 1.
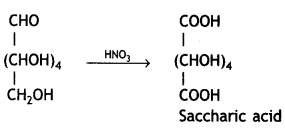
Write the structure of the product obtained when glucose is oxidised with nitric acid. (CBSE 2012)
Answer:
Saccharic acid (or glucaric acid) is formed.
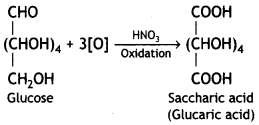
Question 2.
Write a reaction that shows that all the carbon atoms in glucose are linked in a straight chain. (CBSE 2012)
Answer:
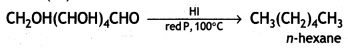
When glucose is heated with HI and red P at 100°C for a long period, it gives n-hexane.
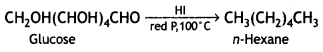
The formation of n-hexane suggests that all six carbon atoms in glucose are arranged in a straight chain.
Question 3.
What are the hydrolysis products of sucrose? (CBSE 2019C)
Answer:
Glucose and fructose
![]()
Question 4.
Write the name of the linkage joining two amino acids. (CBSE 2013)
Answer:
Peptide linkage
Question 5.
What are the hydrolysis products of lactose? (CBSE2019C)
Answer:
Lactose on hydrolysis gives β – D – galactose and β – D – glucose.
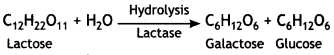
Question 6.
What are three types of RNA molecules which perform different functions? (CBSE Delhi 2013)
Answer:
(i) messenger RNA: m – RNA
(ii) ribosomal RNA: r – RNA
(iii) transfer RNA: t – RNA
Question 7.
What type of bonding helps in stabilising the a-helix structure of proteins? (CBSE Delhi 2013)
Answer:
Hydrogen bonding between -NH and -C = 0 groups of peptide bonds.
Question 8.
What is a glycosidic linkage? (CBSE Delhi 2013)
Answer:
Two monosaccharide units are linked to each other by a bond called glycosidic linkage.
Question 9.
Which of the two components of starch is water-soluble? (CBSE Delhi 2014)
Answer:
Amylose
Question 10.
Which component of starch is a branched polymer of a-glucose and insoluble in water? (CBSE Delhi 2014)
Answer:
Amylopectin
Question 11.
What are the products of hydrolysis of maltose? (CBSE 2014)
Answer:
Glucose
![]()
Question 12.
What is the basic structural difference between glucose and fructose?
OR
Write the products obtained after hydrolysis of lactose. (CBSE Delhi 2019)
Answer:
Glucose contains an aldehyde group (-CHO) and is aldose, which is present at the end of the carbon chain, i.e. C1.
Fructose contains a keto group (>C = 0) and is called ketose, which is present at a carbon atom next to the terminal, i.e. C2.
OR
Hydrolysis of lactose gives β – D – glucose and β – D – galactose.
![]()
Question 13.
What is the difference between a glycosidic linkage and a peptide linkage?
OR
What is the difference between Nucleotide and Nucleoside? (CBSE AI 2019)
Answer:
Glycosidic linkage is the linkage that joins two monosaccharides through an oxygen atom (-0-). Peptide linkage is the linkage that joins two amino acids through -CONH- bond.
OR
The molecules in which one of the nitrogen bases (purines or pyrimidine) is bonded with a sugar molecule (ribose or deoxyribose) is a nucleoside. The nucleoside linked with the phosphate group is called a nucleotide.
Question 14.
What is meant by the inversion of sugar? (CBSE Sample Paper 2011)
Answer:
The change of specific rotation of sugar from dextrorotatory to laevorotatory is called inversion of sugar.
Question 15.
Glucose does not give 2, 4-DNP test and Schiff’s test. Why? (CBSE Sample Paper 2011)
Answer:
Glucose has a cyclic structure in which the -CHO group is not free because it forms a hemiacetal linkage with the -OH group at C-5. Therefore, it does not give 2, 4-DNP test although it has -CHO group.
Question 16.
Write any two reactions of glucose that could not be explained by an open-chain structure of the glucose molecule.
Answer:
The open-chain structure of a glucose molecule cannot explain the following:
- Glucose does not react with sodium bisulphite (NaHS03) to form an addition product though it has an aldehyde group,
- Glucose does not give the Schiffs test and 2,4-DNP test like other aldehydes.
Question 17.
Write the product when D-glucose reacts with the cone. HNO3.
Answer:
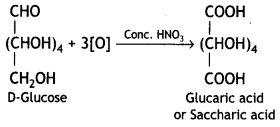
Question 18.
(i) Which vitamin deficiency causes rickets?
Answer:
Vitamin D
(ii) Name the base that is found in the nucleotide of RNA only. (CBSE Sample Paper 2017-18)
Answer:
Uracil
Question 19.
Write the Zwitter ion structure of glycine. (CBSE Sample Paper 2011)
Answer:
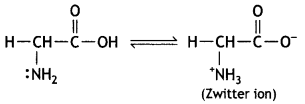
Question 20.
Name a carbohydrate present in the liver, muscles and brain. (CBSE 2019C)
Answer:
Glycogen
Question 21.
Name the species formed when an aqueous solution of amino acid is dissolved in water. (CBSE Sample Paper 2019)
Answer:
Zwitter ion/dipolar ion
Question 22.
Name the unit formed by the attachment of a base to the 1′ position of sugar in a nucleoside. (CBSE Sample Paper 2019)
Answer:
Nucleotide
Biomolecules Important Extra Questions Short Answer Type
Question 1.
Name the bases present in RNA. Which one of these is not present in DNA? (CBSE Delhi 2011)
Answer:
- The bases present in RNA are guanine, adenine, cytosine and uracil.
- Uracil is not present in DNA. Instead, it contains thymine.
Question 2.
What is meant by biocatalysts? (CBSE Delhi 2012)
Answer:
Biocatalysts. Biocatalysts or enzymes are produced by living cells which catalyse the biochemical reactions occurring in living cells.
Question 3.
Name one fibrous protein and one globular protein.
Answer:
Fibrous: Keratin Globular: Albumin
Question 4.
What is the chemical name of vitamin A and which disease is caused by its deficiency?
Answer:
Retinol, deficiency disease: Xerophthalmia.
Question 5.
What is the chemical name of vitamin C and which disease is caused by its deficiency?
Answer:
Ascorbic acid, deficiency disease: Scurvy.
Question 6.
Which enzyme is present in saliva? What is its function?
Answer:
The enzyme present in the saliva is amylase. It hydrolyses starch into maltose.
Question 7.
What is the difference between DNA and RNA on the basis of the bases they contain?
Answer:
Both DNA and RNA contain two bases derived from purine: guanine and adenine and one base derived from pyrimidine: cytosine. However, they have a fourth different base; DNA contains thymine whereas RNA contains uracil.
Question 8.
Shanti, a domestic helper of Mrs Anuradha, fainted while mopping the floor. Mrs Anuradha immediately took her to the nearby hospital where she was diagnosed to be severely ‘anaemic’. The doctor prescribed an iron-rich diet and multivitamins supplement to her. Mrs Anuradha supported her financially to get the medicines. After a month, Shanti was diagnosed to be normal.
After reading the above passage, answer the following questions:
(i) What values are displayed by Mrs Anuradha?
Answer:
Mrs Anuradha has shown generosity and a caring attitude for the health and life of her domestic helper. Timely help, kindness and financial support saved her domestic helper from a serious health problem.
(ii) Name the vitamin whose deficiency causes ‘pernicious anaemia’.
Answer:
B12
(iii) Give an example of a water-soluble vitamin. (CBSE 2013)
Answer:
Vitamin C
Biomolecules Important Extra Questions Long Answer Type
Question 1.
Differentiate between the following:
(i) Amylose and Amylopectin
(ii) Peptide linkage and Glycosidic linkage
(iii) Fibrous proteins and Globular proteins
OR
Write chemical reactions to show that the open structure of D-glucose contains the following:
(i) Straight chain
(ii) Five alcohol groups
(iii) Aldehyde as carbonyl group (CBSE Delhi 2019)
Answer:
(i) Amylose and Amylopectin:
| Amylose | Amylopectin |
| 1. It consists of a long chain of a-D- glucose. | 1. It consists of branched polymeric chains of a-D- glucose. |
| 2. It is water-soluble. | 2. It is water-insoluble. |
(ii) Peptide linkage and Glycosidic linkage:
| Peptide linkage | Glycosidic linkage |
| The linkage joins two amino acids through -CONH- bond. | The linkage joins two monosaccharides through an oxygen atom(-o). |
(iii) Fibrous and Globular proteins:
| Fibrous | Globular |
| 1. The polypeptide chains run parallel and are held by hydrogen and sulphide bonds. | 1. The polypeptide chains coil around to give a spherical shape. |
| 2. These are insoluble in water. | 2. These are soluble in water. |
| 3. For example, Keratin in hair. | 3. For example, egg albumin. |
OR
(i) Glucose on prolonged heating with HI and red P at 100 °C gives n-hexane, which shows the straight-chain structure of glucose.
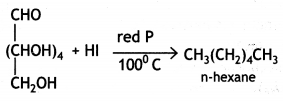
(ii) Glucose reacts with acetic anhydride in the presence of anhydrous zinc chloride to form glucose pentaacetate. The formation of pentaacetate confirms the presence of five -OH groups in the glucose molecule.
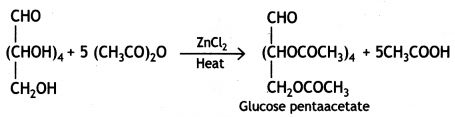
(iii) Glucose reacts with hydrogen cyanide forming glucose cyanohydrin. This shows that glucose contains a carbonyl group (>C=0).
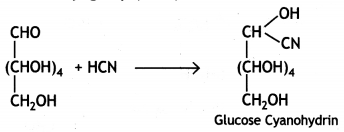
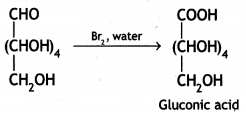
Glucose gets oxidised to six carbon carboxylic acid (gluconic acid) on treatment with a mild oxidising agent like Br2 water. This indicates that the carbonyl group present in glucose is an aldehyde (-CHO) group.
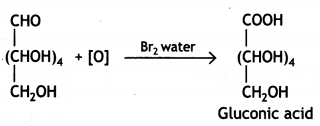
Question 2.
Define the following with a suitable example in each:
(i) Oligosaccharides
(ii) Denaturation of protein
(iii) Vitamins
OR
Write the reactions involved when D-glucose is treated with the following reagents:
(i) Br2 water
(ii) H2N-OH
(iii) (CH3CO)20 (CBSE Delhi 2019)
Answer:
(i) Oligosaccharides. These are the carbohydrates that give two to ten monosaccharide molecules on hydrolysis, for example, disaccharides such as sucrose, lactose, maltose (C12H22O16), trisaccharides such as raffinose (C18H32O16).
(ii) Deriaturatlon of proteins: A process that changes the physical and biological properties of proteins without affecting the chemical composition of the protein is called denaturation. It is caused by certain physical change like change In temperature or chemical change like change In pH presence of electrolytes, etc., for example, coagulation of albumin present in the white of an egg.
(iii) Vitamins: The organic compounds which cannot be produced by the body and must be supplied in small amounts in the diet to perform specific biological functions for the normal health, growth and maintenance of the body are called vitamins, for example, vitamin A, B, C, D, etc.
OR
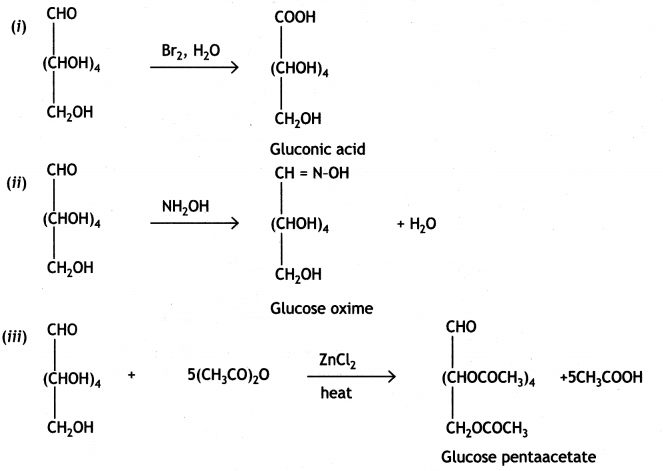
Question 3.
Define the following terms with a suitable example of each:
(a) Anomers
Answer:
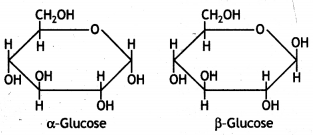
Anomers: Carbohydrates that differ in configuration at the glycosidic carbon (i.e. C1 in aldoses and C2 in ketoses) are called anomers. For example, α-D-glucose and β-D- glucose.
(b) Essential amino acids
Answer:
Essential amino acids: The amino acids which cannot be made by our bodies and must be supplied in our diet are called essential amino acids. For example, valine, leucine, etc.
(c) Denaturation of protein (CBSE AI 2019)
Answer:
A process that changes the native conformation of a protein is called denaturation. The denaturation can be caused by changes in pH, temperature, presence of salts of certain chemical agents. The denatured protein will lose its biological activity. During denaturation, the protein molecule uncoils from an ordered and specific conformation into a more random conformation and protein precipitates from the solution. For example, when an egg is boiled in water, the globular proteins present in it change to a rubber-like insoluble mass.
Question 4.
Define the following terms with a suitable example of each:
(a) Tertiary structure of the protein
Answer:
The tertiary structure of proteins: Tertiary structure of proteins arises because of the folding, coiling or bending of polypeptide chains producing a three-dimensional structure. This structure gives the overall shape of proteins. For example, fibrous proteins such as silk, collagen and α-keratin have large helical structures
(b) Essential amino acids
Answer:
Essential amino acids: The amino acids which cannot be synthesised in our body and must be supplied through our diet are called essential amino acids, for example, valine, leucine, etc.
(c) Disaccharides (CBSE AI 2019)
Answer:
Disaccharides: The carbohydrates which on hydrolysis give two same or different monosaccharides are called disaccharides. Sucrose is an example of a disaccharide, which on hydrolysis gives glucose and fructose.
Question 5.
Describe what do you understand by primary structure and secondary structure of proteins. (CBSE Delhi 2011)
Answer:
Primary structure:
The sequence in which the amino acids are linked in one or more polypeptide chains of a protein is called the primary structure.
The primary structure is usually determined by its successive hydrolysis with enzymes or mineral acids.
Secondary structure: The secondary structure gives the manner in which the polypeptide chains are folded or arranged. Therefore, it gives the shape or conformation of the protein molecule. This arises from the plane geometry of the peptide bond and hydrogen bond between the —C=O and N—H groups of different peptide bonds. Pauling and Corey studied that there are two common types of structures:
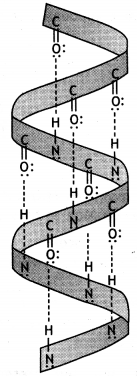
(i) α – Helix structure: In this structure, the formation of hydrogen bonding between amide groups within the same chain causes the peptide chains to coil up into a spiral structure (Fig. 1). This is called the a-helix.
(ii) β-pleated sheet structure: In this structure, all polypeptide chains are stretched out to nearly maximum extension and then laid side by side in a zigzag manner to form a flat sheet. Each chain is held to the two neighbouring chains by a hydrogen bond. These sheets are stacked one upon another to form a three-dimensional structure called
β-pleated sheet structure (Fig. 2).
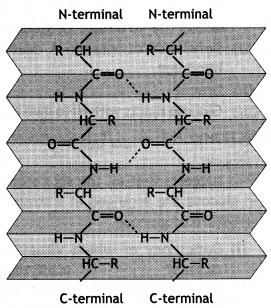
Question 6.
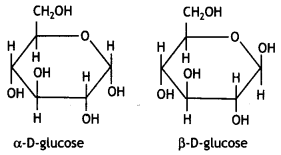
ExplaIn what is meant by the pyranose structure of glucose. (CBSE 2011)
Answer:
The six-membered cyclic structure of glucose is catted pyranose structure similar to the pyran heterocyclic compound. For structure,
Question 7.
Write the main structural difference between RNA and DNA. Of the four bases, name those which are common to both DNA and RNA. (CBSE 2011)
Answer:
Structural differences:
| DNA | RNA |
| (i) It has a double-stranded a-helix structure in which two strands are coiled spirally in opposite directions. | (i) It has a single-stranded a-helix structure. |
| (ii) The sugar molecule present in DNA is 2-deoxyribose. | (ii) The sugar molecule present in RNA is ribose. |
| (iii) Nitrogenous base uracil is not present. | (iii) Nitrogenous base thymine is not present. |
Common bases present in both: adenine, cytosine and guanine.
Question 8.
(i) Deficiency of which vitamin causes night blindness? (CBSE DelhI 2014)
(ii) Name the base that is found in the nucleotide of RNA only.
(iii) Glucose on reaction with HI gives n-hexane. What does It suggest about the structure of glucose?
Answer:
(i) Deficiency of Vitamin A causes night blindness.
(ii) Uracil
(iii) Glucose reacts with HI to give n-hexane.
![]()
This suggests that alt the six carbon atoms in glucose are arranged in a straight chain structure of glucose.
Question 9.
(i) DefIciency of which vitamin causes rickets? (CBSE Delhi 2014)
Answer:
Vitamin D.
(ii) Give an example for each of fibrous protein and globular protein.
Answer:
Fibrous protein: Keratin Globular protein: Albumin
(iii) Write the product formed on reaction of D-glucose with Br2 water.
Answer:
Question 10.
(i) Deficiency of which vitamin causes scurvy? (CBSE DelhI 2014)
Answer:
Vitamin C.
(ii) What type of linkage is responsible for the formation of proteins?
Answer:
Peptide Linkage.
(iii) Write the product formed when glucose is treated with HI.
Answer:
Question 11.
Define the following terms: (CBSE 2014)
(i) Gtycosidic linkage
Answer:
Glycosidic linkage: The condensation of hydroxyl groups of two monosaccharides to form a link between them is called glycosidic linkage.
(ii) Invert sugar
Answer:
Invert sugar: The sugar which on hydrolysis with dilute adds or enzymes gives mixture having specific rotation opposite to the original is called invert sugar. For example, sucrose is inverted sugar.
(iii) Oligosaccharides
Answer:
Oligosaccharides: These are the carbohydrates that give two to ten monosaccharide molecules on hydrolysis. These are further classified as disaccharides, trisaccharides, tetrasaccharides, etc. depending upon the number of monosaccharide units present in their molecules. For example, Disaccharides: Sucrose, lactose, maltose. All these have the molecular
Formula C12H22O11.
Trisaccharide: Raffinose (C18H32O16).
Tetrasaccharides: Stachyose (C24H42O21 ).
Question 12.
Define the following terms: (CBSE 2014)
(i) Nucleotide
Answer:
Nucleotide: A unit formed by the combination of a nitrogen-containing heterocyclic base, a pentose sugar and a phosphoric acid group.
(ii) Anomers
Answer:
Anomers: The anomers are the isomers formed due to the change in the configuration of the -OH group at C-1 of glucose. For example, α-and β-forms of glucose are anomers.
(iii) Essential amino acids.
Answer:
Essential amino acids: The amino acids which cannot be made by our bodies and must be supplied in our diet for the growth of the body are called essential amino acids.
Question 13.
Differentiate between the following:
(a) Fibrous protein and Globular protein
Answer:
Difference between fibrous protein and globútar protein:
| Fibrous | Globular |
| (i) The polypeptide chains run parallel and are held together by hydrogen and disulphide bonds. | 1. The polypeptide chains colt around to give a spherical shape. |
| (ii) These are insoluble in water. | 2. These are soluble in water. |
| (iii) For example, keratin in hair | 3. For example, albumin in egg. |
(b) Essential amino acids and Non-essential amino acids
Answer:
Difference between essential amino acids and non-essential amino acids:
| Essential amino acids | Non-essential amino acids |
| These are not synthesìsed in our body and must be supplied in the diet. | These are synthesised in our body and not required in our diet. |
| For example, valine. | For example, alanine |
(C) Amylose and Amylopectin (CBSE AI 2019)
Answer:
Difference between amylose and amylopectin:
| Amylose | Amylopectin |
| It consists of branched polymeric chains of α – D – glucose. | It consists of a long straight chain of α – D – glucose. |
| It is water-insoluble. | It is water-soluble. |
Question 14.
(i) Which one of the following is a polysaccharide: (CBSE 2015, 2014)
Starch, Maltose, Fructose, Glucose
Answer:
Starch
(ii) What is the difference between native protein and denatured protein?
Answer:
Native protein is the protein found in a biological system with a unique three-dimensional structure and biological activity.
Denatured protein is the protein which loses its biological activity by certain physical or chemical treatment such as changes in pH, temperature, presence of certain salts or chemical agent, known as denaturation.
(iii) Write the name of the vitamin responsible for the coagulation of blood.
Answer:
Vitamin K.
Question 15.
(i) Write one reaction of D-glucose which cannot be explained by its open-chain structure. (CBSE 2016)
Answer:
Despite having an aldehydic (-CHO) group, glucose does not react with NaHS03 to form an addition product.
(ii) What type of linkage is present in nucleic acids?
Answer:
Phosphodiester linkage
(iii) Give one example each for water-soluble vitamins and fat-soluble vitamins.
Answer:
Water-soluble vitamin: vitamin B Fat-soluble vitamin: vitamin A
Question 16.
What is essentially the difference between the α-form of D-glucose and β-form of D-glucose? Explain.
Answer:
α-form and β-form of glucose differ in the orientation of —H and —OH groups around the C1 atom. The isomer having the -OH group on the right is called α-D-glucose while the one having -OH group on the left is called β-D-glucose. Such pairs of optical isomers which differ in the configuration only around C1 are called anomers. The structures of these two may be shown below:
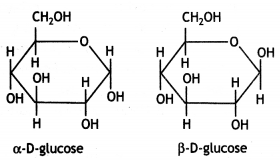
These two forms are crystalline and have different melting points and optical rotations. For example, α-form of glucose has m.p. 419 K and |α|D = +111° and the β-form of glucose has m.p. 423 K and |α|D = +19.2°.
Question 17.
(a) What happens when D-glucose is treated with the following reagents:
(i) HI
Answer:
When glucose is treated with HI, it forms n-hexane, suggesting that all the six carbon atoms are linked in a straight line.
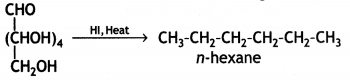
(ii) cone. HN03
Answer:
Glucose on treatment with nitric acid gives a dicarboxylic acid, saccharic acid.
(b) What is the basic structural difference between starch and cellulose? (CBSE 2019C)
Answer:
Starch consists of two components: amylose and amylopectin. Amylose is a long linear polymer of 200-1000 a-D-(+)-glucose units held by C1 – C4 glycosidic linkages. It is soluble in water. Amylopectin is a branched-chain polymer of a-D-(+)-glucose linkages whereas branching occurs by C1 – C6 glycosidic linkage. It is insoluble in water.
On the other hand, cellulose is a straight-chain polysaccharide composed only of (β – D – (+)-glucose units which are formed by the glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
Question 18.
(1) Write the name of two monosaccharides obtained on hydrolysis of lactose sugar. (CBSE Delhi 2016)
Answer:
Glucose, Galactose
(ii) Why Vitamin C cannot be stored in our body?
Answer:
Vitamin C is water-soluble and hence cannot be stored in our body.
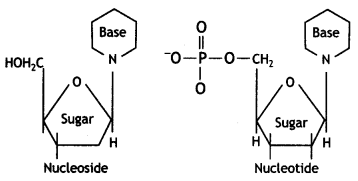
(iii) What is the difference between a nucleoside and a nucleotide?
Answer:
A nucleoside contains only two basic components of nucleic acids, namely a pentose sugar and a nitrogenous base.
A nucleotide contains all the three basic components of nucleic acids, namely a phosphoric acid group, a pentose sugar and a nitrogenous base.
Question 19.
Define the following with an example of each: (CBSE 2018)
(i) Polysaccharides
(ii) Denatured protein
(iii) Essential amino acids
OR
(i) Write the product when D-glucose reacts with the cone. HNO3.
(ii) Amino acids show amphoteric behaviour. Why?
(iii) Write one difference between a-helix and p-pleated structures of proteins.
Answer:
(i) Carbohydrates that give a large number of monosaccharide units on hydrolysis or a large number of monosaccharide units joined together by glycosidic linkage, e.g. starch, glycogen, cellulose.
(ii) Proteins that lose their biological activity or proteins in which secondary and tertiary structures are destroyed, e.g. curdling of milk.
(iii) Amino acids cannot be synthesised in the body. e.g. Valine / Leucine
OR
(i) Saccharic acid / COOH-(CHOH)4-COOH
(ii) Due to the presence of carboxyl and amino group in the same molecule or due to formation of zwitterion or dipolar ion.
(iii) a-helix has intramolecular hydrogen bonding while p-pleated has intermolecular hydrogen bonding / a-helix results due to regular coiling of polypeptide chains while in p-pleated all polypeptide chains are stretched and arranged side by side.
Question 20.
Explain the following:
(i) Amino acids behave like salts rather than simple amines or carboxylic acids. (CBSE 2018C)
Answer:
Due to the formation of zwitterion.
(ii) The two strands of DNA are complementary to each other.
Answer:
The two strands are complementary to each other because the hydrogen bonds are formed between specific pairs of bases.
(iii) Reaction of glucose indicates that the carbonyl group is present as an aldehydic group in the open structure of glucose.
Answer:
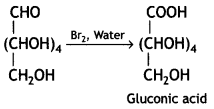
Glucose gets oxidised to gluconic acid on reaction with a mild oxidising agent like Bromine water.
Question 21.
What is essentially the difference between α-glucose and β-glucose? What is meant by the pyranose structure of glucose?
Answer:
α-form and β-form of glucose differ in the orientation of -H and -OH groups around C, atom. The isomer having the -OH group on the right is called α-D-glucose while the one having -OH group on the left is called β-D-glucose. Such pairs of optical isomers which differ in the configuration only around C1 are called anomers. The structures of these two may be shown below:
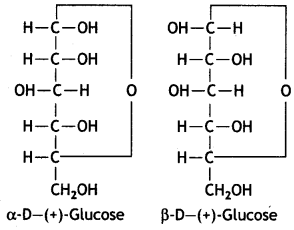
These two forms are crystalline and have different melting points and optical rotations. For example, α-form of glucose has m.p. 419 K and |α|D = + 111° and the β-form of glucose has m.p. 423 K and |α|D = + 19.2°.
The α-D-glucose and β-D-glucose can be drawn in a simple six-membered ring form called pyranose structures. These resemble pyran which is a six-membered heterocyclic ring containing five carbon atoms and one oxygen atom.
These are known as pyranose structures and are shown below:
Question 22.
Which sugar is called invert sugar? Why is it called so?
Answer:
Sucrose is called invert sugar. The sugar obtained from sugar beet is a colourless, crystalline and sweet substance. It is very soluble in water and its aqueous solution is dextrorotatory having [α]D = + 66.5°.
On hydrolysis with dilute acids or enzyme invertase, cane sugar gives an equimolar mixture of D-(+)-glucose and D-(-)-fructose.
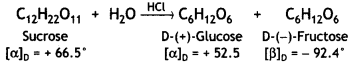
So, sucrose is dextrorotatory but after hydrolysis, gives dextrorotatory glucose and laevorotatory fructose. D-(-)-fructose has a greater specific rotation than D-(+)- glucose. Therefore, the resultant solution upon hydrolysis is laevorotatory in nature with a specific rotation of (-39.9°). Since there is a change in the sign of rotation from Dextro before hydrolysis to Laevo after hydrolysis, the reaction is called Inversion reaction and the mixture (glucose and fructose) is called invert sugar.
Question 23.
How do you explain the presence of an aldehydic group in a glucose molecule?
Answer:
Glucose reacts with hydroxylamine to form a monoxime and adds one molecule of hydrogen cyanide to give cyanohydrin.
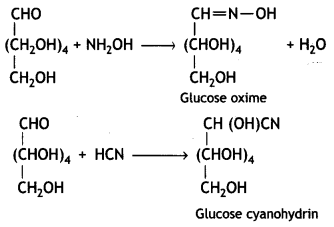
Therefore, it contains a carbonyl group which can be an aldehyde or a ketone. On mild oxidation with bromine water, glucose gives gluconic acid which is a carboxylic acid-containing six carbon atoms.
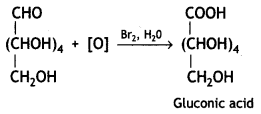
This indicates that the carbonyl group present in glucose is an aldehydic group.
Question 24.
Describe the term D- and L-configuration used for sugars with examples.
Answer:
The sugars are divided into two families: the D-family and L-family which have definite configurations. These configurations are represented with respect to glyceraldehyde as the standard. The glyceraldehyde may be presented in two forms:
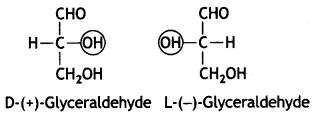
The D-configuration has —OH attached to the carbon adjacent to —CH2OH on the right while L-configuration has —OH attached to the carbon adjacent to —CH2OH on left.
The sugars are calLed D- or L- depending upon whether the configuration of the molecule is related to D-glyceraldehyde or L-glyceraldehyde. It has been found that all naturally occurring sugars beLong to D-series, e.g. D-glucose, D-ribose and D-fructose.