Here we are providing Class 12 Chemistry Important Extra Questions and Answers Chapter 5 Surface. Class 12 Chemistry Important Questions are the best resource for students which helps in Class 12 board exams.
Class 12 Chemistry Chapter 5 Important Extra Questions Surface Chemistry
Surface Chemistry Important Extra Questions Very Short Answer Type
Question 1.
Give an example of a shape selective catalyst. (CBSE Delhi 2010, 2011)
Answer:
Zeolite catalyst known as ZSM-5.
Question 2.
Why are medicines more effective in colloidal state? (CBSE Delhi 2019)
Answer:
Medicines are most effective in colloidal state because colloids have large surface area and hence the medicines can be easily adsorbed and assimilated.
Question 3.
What is difference between an emulsion and a gel? (CBSE Delhi 2019)
Answer:
Emulsions are colloidal solutions in which both the dispersed phase and the dispersion medium are liquids. Gel is a colloidal system in which a liquid is dispersed in a solid.
Question 4.
Define ‘peptization’. (CBSE2012)
Answer:
The process of converting a freshly prepared precipitate into colloidal form by addition of a suitable electrolyte is called peptization.
Question 5.
What is meant by ‘shape-selective catalysis? (CBSE 2012)
Answer:
The catalytic reaction which depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape selective catalysis.
Question 6.
What happens when a freshly precipitated
Fe(OH)3 is shaken with little amount of dilute solution of FeCl3? (CBSE 2013)
Answer:
A reddish brown colloidal solution of Fe(OH)3 is obtained. This process is called peptization.
Question 7.
What is especially observed when a beam of light is passed through a colloidal solution? (CBSE 2013)
Answer:
Tyndall effect
Question 8.
Give one example each of sol and gel. (CBSE Delhi 2014)
Answer:
Sol: As2S3 sol.
Gel: Cheese.
Question 9.
Give one example each of ‘oil in water’ and ‘water in oil’ emulsion. (CBSE 2014)
Answer:
Oil in water emulsion:
Milk Water in oil emulsion: Butter
Question 10.
Write a method by which lyophobic colloids can be coagulated. (CBSE 2015)
Answer:
By electrophoresis lyophobic colloids can be coagulated. Other methods are by mixing two oppositely charged sols or by boiling or by addition of electrolyte.
Question 11.
Write the main reason for the stability of colloidal sols. (CBSE Delhi 2016)
Answer:
The stability of colloidal sols is because of the presence of electrical charge on the particles which prevents the particles to come close together.
Question 12.
What are lyophobic colloids? Give one example for them. (CBSE Delhi 2011)
Answer:
The colloidal solutions in which the particles of the dispersed phase have no affinity for the dispersion medium are called lyophobic colloids. For example, metal sulphides like As2S3.
Question 13.
Write one similarity between physisorption and chemisorption. (CBSE Delhi 2017, CBSE AI 2017)
Answer:
Both are surface phenomena and increase with increase in surface area.
Question 14.
Why are lyophilic colloidal sols more stable than lyophobic colloidal sols? (CBSE Delhi 2015)
Answer:
The lyophilic colloidal sols are more stable because they are highly hydrated in solution.
Question 15.
What is the difference between a sol and a gel? (CBSE Delhi 2017)
Answer:
In a sol, dispersion medium is liquid and dispersed phase is solid. On the other hand, in a gel, dispersion medium is solid and dispersed phase is liquid.
Question 16.
What type of colloid is formed when a liquid is dispersed in a solid? Give an example. (CBSE AI 2017)
Answer:
Gel
For example: Cheese
Question 17.
Which of the following is most effective electrolyte in the coagulation of Fe2O3.x H2O/Fe3+ sol?
KCl, AlCl3, MgCl2, K4[Fe(CN)6] (CBSE Sample Paper 2011)
Answer:
Since Fe(OH)3 sol is positively charged, the anion having highest charge will be most effective, i.e. [Fe(CN)6]4.
Question 18.
Why is colloidal gold used for intramuscular injection? (CBSE Sample Paper 2011)
Answer:
Colloidal gold is more effective because of larger surface area and therefore, is easily assimilated with blood which is colloidal.
Question 19.
What is colloidion? (CBSE Sample Paper 2011)
Answer:
4% solution of nitrocellulose in a mixture of alcohol and ether.
Question 20.
Leather gets hardened after tanning. Why? (CBSE Delhi 2015)
Answer:
Animal hide is colloidal in nature and has positively charged particles. When it is soaked in tannin which has negatively charged colloidal particles, it results in mutual coagulation. This results in the hardening of leather.
Question 21.
It is necessary to remove CO when ammonia is prepared by Haber’s process. Explain. (CBSE Delhi 2015)
Answer:
Carbon monoxide acts as a poison for the catalyst in Haber’s process and therefore, it will lower the activity of the catalyst. Thus, CO must be removed when ammonia is obtained by Haber’s process.
Question 22.
Addition of alum purifies water. Why? (CBSE AI 2015)
Answer:
Alum coagulates the impurities present in water by neutralising the charge.
Question 23.
Out of sulphur sol and proteins, which one forms multimolecular colloids? (CBSE Delhi 2016)
Answer:
Proteins are macromolecules which cannot form multimolecular colloids while sulphur sol has smaller S8 molecules which can coagulate to form multimolecular colloids.
Question 24.
Write the chemical method by which Fe(OH)3 sol is prepared from FeCl3. (CBSE AI 2017)
Answer:
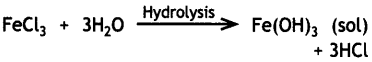
Question 25.
Out of MgCl2 and AlCl3 which one is more effective in causing coagulation of negatively charged sol and why? (CBSE Delhi 2016)
Answer:
According to Hardy-Schulze rule, for negatively charged sol, greater the valency of the positive ion of the electrolyte added, greater is its coagulating power. Thus, AlCl3 (Al3+ ions) is more effective in causing coagulation of negatively charged sol than MgCl2 (Mg2+ ions).
Question 26.
Give one example each of lyophobic sol and lyophilic sol. (CBSE Delhi 2014)
Answer:
Lyophobic : As2S3, Lyophilic : Gelatin
Question 27.
Out of BaCl2 and KCl which one is more effective in causing coagulation of negatively charged colloidal sol. Give reason. (CBSE Delhi 2015)
Answer:
BaCl2 because greater the valency of the coagulating ion (positive ion), greater is its tendency to coagulate.
Question 28.
What are the dispersed phase and . dispersion medium in milk? (CBSE AI 2014)
Answer:
Liquid (dispersed phase), Liquid (dispersion medium)
Question 29.
What type of colloid is formed when a solid is dispersed in liquid? Give an example. (CBSE AI 2017)
Answer:
Sol, paints
Question 30.
CO (g) and H2(g) react to give different products in the presence of different catalysts. Which ability of the catalyst is shown by these reactions? (CBSE AI 2018)
Answer:
Selectivity of a catalyst. It is the ability of a catalyst to selectively form a particular product.
Surface Chemistry Important Extra Questions Short Answer Type
Question 1.
Describe a conspicuous change observed when
(i) a solution of NaCl is added to a sol of hydrated ferric oxide.
(ii) a beam of light is passed through a solution of NaCl and then through a sol. (C.B.S.E. Delhi 2012)
Answer:
1. Hydrated ferric oxide is positively charged. When an electrolyte such as NaCl is added, the excess Cl– ions neutralise positive charge and cause coagulation of the sol.
2. When a beam of light is passed through a colloidal solution, the path of the light becomes visible when viewed from the direction at right angle to that of incident beam. This phenomenon is called Tyndall effect. This is because of scattering of light by colloidal particles. The particles first absorb the incident light and then a part of it gets scattered by them and therefore the part becomes visible when seen at right angle to the direction of incident beam.
Question 2.
Name the two groups into which phenomenon of catalysis can be divided. Give an example of each group with the chemical equation involved. (CBSE Delhi 2012)
Answer:
Catalysis can be divided into two groups
(i) Homogeneous catalysis
(ii) Heterogeneous catalysis
(i) Homogenous catalysis: When the catalyst is present in the same phase as the reactants and products, it is called homogeneous catalysis. For example,
![]()
SO2(g) is oxidised to SO3(g) in the presence of gaseous nitric oxide as catalyst.
(ii) Heterogeneous catalysis: When the catalyst is in different phase than the
reactants, it is called heterogeneous catalysis. For example,
![]()
Question 3.
Write the dispersed phase and dispersion medium of the following colloidal systems: (CBSE Delhi 2013)
(i) Smoke
(ii) Milk
Answer:
Colloidal system: Dispersed phase/Dispersion medium
(i) Smoke: Solid/Gas
(ii) Milk: Liquid/Liquid
Question 4.
What are lyophilic and lyophobic colloids? Which of these sols can be easily coagulated on the addition of small amounts of electrolytes? (CBSE Delhi 2013)
Answer:
The colloidal solutions in which the particles of the dispersed phase have great affinity for the dispersion medium are called lyophilic sols. For example, glue, gelatin, starch, proteins, etc.
The colloidal solutions in which there is no affinity between the particles of the dispersed phase and the dispersion medium are called lyophobic sols. For example, solutions of . metals like Ag, Au, Al(OH)3, Fe(OH)3, etc.
Lyophobic sols can be easily coagulated on the addition of small amounts of electrolytes.
Question 5.
What is the difference between oil/water (O/W) type and water/oil (W/O) type emulsions? Give an example of each type. (CBSE Delhi 2013)
Answer:
1. Oil-in-water emulsion (o/w). In this case, oil acts as the dispersed phase (small amount) and water as the dispersion medium (excess), e.g. milk is an emulsion of soluble fats in water and here casein acts as an emulsifier. Vanishing cream is another example of this class. Such emulsions are called aqueous emulsions.
2. Water-in-oil emulsion (w/o). In this case, water acts as the dispersed phase while the oil behaves as the dispersion medium, e.g. butter, cod liver oil, cold cream, etc. Such types of emulsions are called oily emulsions.
Question 6.
Peptizing agent is added to convert precipitate into colloidal solution. Explain. (CBSE Sample Paper 2011)
Answer:
Peptization is a process of converting a freshly prepared precipitate into colloidal form by the addition of an electrolyte called peptizing agent. The suitable ions from the peptizing agent (electrolyte) are adsorbed by the particles of the precipitate giving it positive or negative charge. The charged particles repel one another and break up the precipitate into smaller particles of the size of the colloid. Therefore, it results into the formation of colloid.
For example, on treating a precipitate of iron (III) oxide with a small amount of FeCl3 solution gives a reddish brown coloured colloidal solution.
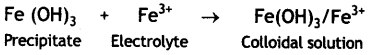
Question 7.
Cottrell’s smoke precipitator is fitted at the mouth of chimney used in factories. Give reasons. (CBSE Sample Paper 2011)
Answer:
Smoke coming out of chimney of a factory is a colloidal solution of solid carbon particles which are charged in nature.
The mouth of the chimneys used in factories is fitted with Cottrell smoke precipitator. In this method, the smoke is allowed to pass through a chamber having a series of plates charged to very high potential (20,000 to 70,000 V).
Charged particles of smoke get attracted by charged plates, get precipitated and the gases coming out of chimney become free of charged carbon and dust particles.
Question 8.
Differentiate between peptization and coagulation.
(CBSE Sample Paper 2011, CBSE AI 2017)
Answer:
Peptization is the process of converting a freshly prepared precipitate into collodial form by the the addition of a suitable electrolyte. The electrolytes used for the purpose are called peptizing agents. On the other hand, coagulation is the phenomenon of precipitation of a collodial solution by the addition of excess of an electrolyte.
Question 9.
Explain:
(i) Sky appears blue in colour.
(ii) A freshly formed precipitate of ferric hydroxide can be converted to a colloidal sol by shaking it with a small quantity of ferric chloride. (CBSE Sample Paper 2011)
Answer:
1. Dust particles along with water suspended in air scatter blue light which reaches our eyes and therefore, sky looks blue to us.
2. When we add FeCl3 to a freshly formed precipitate of Fe(OH)3, peptization occurs. The Fe3+ ions are adsorbed on the surface of the precipitate which ‘ ultimately breaks down into smaller particles of colloidal size.
Question 10.
What happens when
(i) a freshly prepared precipitate of Fe(OH)3 is shaken with a small amount of FeCl3 solution?
(ii) persistent dialysis of a colloidal solution is carried out?
(iii) an emulsion is centrifuged? (CBSE 2018)
Answer:
(i) Peptization occurs / Colloidal solution of Fe(OH)3 is formed.
(ii) Coagulation occurs
(iii) Demulsification or breaks into constituent liquids
Surface Chemistry Important Extra Questions Long Answer Type
Question 1.
Classify colloids where the dispersion medium is water. State their characteristics and write one example of each of these classes.
OR
Explain what is observed when
(i) an electric current is passed through a sol.
(ii) a beam of light is passed through a sol.
(iii) an electrolyte (say NaCl) is added to ferric hydroxide sol. (CBSE 2011)
Answer:
These colloids are of two types:
(i) Hydrophilic
(ii) Hydrophobic
Characteristics: In lyophilic colloids, there is affinity between dispersed phase and the dispersion medium while in the lyophobic colloids, there is no affinity rather hatred between the phases.
The main points of distinction between lyophilic and lyophobic sols are summarized below:
| Lyophilic colloids (Hydrophilic) | Lyophobic collodis (Hydrophobic) |
| 1. These are easily formed by direct mixing. | 1. These are formed only by special methods. |
| 2. These are reversible in nature. | 2. These are irreversible in nature. |
| 3. The particles of colloids are true molecules and are big in size. | 3. The particles are aggregates of many molecules. |
| 4. The particles are not easily visible even under ultramicroscope. | 4. The particles are easily detected under ultramicroscope. |
| 5. These are very stable. | 5. These are unstable and require traces of stabilizers. |
| 6. The addition of small amount of electrolytes causes precipitation (called coagulation) of colloidal solution. | 6. The addition of small amount of electrolytes has less effect. Larger quantities of electrolytes are required to cause coagulation. |
| 7. The particles do not carry any charge. The particles may migrate in any direction or even not under the influence of an electric field. | 7. The particles move in a specific direction i.e., either towards anode or cathode depending upon their charge. |
| 8. The particles of colloids are heavily hydrated due to the attraction for the solvent. | 8. The particles of colloids are not appreciably hydrated due to the hatred for the solvent. |
| 9. The viscosity and surface tension of the sols are much higher than that of the dispersion medium. | 9. The viscosity and surface tension are nearly the same as that of the dispersion medium. |
| 10. They do not show Tyndall effect. | 10. They show Tyndall effect. |
Example: Hydrophilic: Starch,
Hydrophobic : Metal sulphide
OR
(i) On passing electric current through colloidal solution, the colloidal particles move towards the oppositely charged electrodes, where they lose their charge and get coagulated. This is electrophoresis process.
(ii) When a beam of light is passed through a colloidal solution, the path of the light becomes visible when viewed from the direction at right angle to that of incident beam. This phenomenon is called Tyndall effect. This is because of scattering of tight by colloidal particles. The particles first absorb the incident light and then a part of it gets scattered by them and therefore the part becomes visible when seen at right angle to the direction of incident beam.
(iii) Hydrated ferric oxide is positively charged. When an electrolyte such as NaCl is added, the excess Cl– ions neutralise positive charge and cause coagulation of the sol.
Question 2.
Explain how the phenomenon of adsorption finds application in each of the following processes:
(i) Production of vacuum
(ii) Heterogeneous catalysis
(iii) Froth floatation process (CBSE Delhi 2011)
Answer:
(i) Production of vacuum: The adsorption of air in liquid air helps to create a high vacuum in a vessel. This process is used in high vacuum instruments such as Dewar flask for storage of liquid air or liquid hydrogen. The remaining traces of air can be adsorbed by charcoal from the vessel evacuated by a vacuum pump to give a very high vacuum.
(ii) Heterogeneous catalysis: The phenomenon of adsorption is useful in the heterogeneous catalysis. Adsorption of reactants on the solid surface of catalysts increases the rate of reaction. The metals such as Fe, Ni, Pt, Pd, etc. are used in the manufacturing processes. Manufacture of ammonia using iron as catalyst (Haber process), manufacture of sulphuric acid by Contact process and use of finely divided nickel in the hydrogenation of oils are excellent examples of heterogeneous catalysis. Its use is based upon the phenomenon of adsorption.
(iii) In froth floatation process: A low grade sulphide ore is concentrated by separating it from silica and other earthly matter by adsorption using pine oil and frothing agent.
Question 3.
What is meant by coagulation of a colloidal solution? Describe briefly any three methods by which coagulation of lyophobic sols can be carried out. (CBSE 2012)
Answer:
The phenomenon of precipitation of a colloidal sol by the addition of excess of an electrolyte is called coagulation of colloidal sol. Coagulation of lyophobic sols can be carried out by the following methods:
1. By addition of an electrolyte: When an electrolyte is added to the sol, colloidal particles take up ions carrying opposite charge from the electrolyte and get neutralised resulting in coagulation.
2. By mutual precipitation: When two oppositely charged sols are mixed in equimolar proportions, they mutually neutralise their charge and both get coagulated.
3. By electrophoresis: When electrophoresis is carried out for a long time, the particles of dispersed phase move towards oppositely charged electrodes, lose their charge and get coagulated.
Question 4.
Explain the following terms giving a suitable example for each:
(i) Aerosol
(ii) Emulsion
(iii) Micelle (CBSE 2012)
Answer:
(i) Aerosol: It is a colloidal dispersion of a liquid in a gas. For example, fog, mist, cloud.
(ii) Emulsion. Emulsions are the colloidal solutions of two immiscible liquids in which the liquid acts as the dispersed phase as well as the dispersion medium. Types of emulsion. These are of two types:
(a) Oil-in-water emulsion. In this case, oil acts as the dispersed phase (small amount) and water as the dispersion medium (excess), e.g. milk is an emulsion of soluble fats in water and here casein acts as an emulsifier. Vanishing cream is another example of this class. Such emulsions are called aqueous emulsions.
(b) Water-in-oil emulsion. In this case, water acts as the dispersed phase while the oil behaves as the dispersion medium, e.g. butter, cod liver oil, cold cream, etc. Such types of emulsions are called oily emulsions.
(iii) Micelle: The particles of colloidal size formed due to aggregation of several ions or molecules with lyophobic as well as lyophilic particles are called micelles. The common micelles system is soap. The micelles behave as normal electrolytes at low concentrations but as colloids at higher concentrations.
Question 5.
Write three distinct features of chemisorption which are not found in physisorption. (CBSE 2012)
Answer:
Characteristics of Chemisorption:
- The forces between the adsorbate molecules and the adsorbent are strong chemical forces similar to chemical bonds. But no chemical bonds exist in physisorption.
- Chemisorption is irreversible in nature but physisorption is reversible.
- Chemisorption forms mono – molecular layers, but in physisorption multimolecular layers are present.
Question 6.
What are the characteristics of the following colloids? Give one example of each.
(i) Multimolecular colloids
(ii) Lyophobic sols
(iii) Emulsions (CBSE 2013)
Answer:
(i) The colloidal solutions in which atoms or smaller molecules of substances (having diameter less than 1 nm) aggregate together to form particles of colloidal dimensions. The particles thus formed are called multimolecular colloids. For example, sols of gold atoms and sulphur (S8) molecules.
(ii) The colloidal solutions in which there in no affinity (or love rather they have hatred) between the particles of the dispersed phase and the dispersion medium are called lyophobic sols.
For example, metal sulphides like As2S3.
(iii) The colloidal solutions in which both the dispersed phase and the dispersion medium are liquids are called emulsions. For example, milk.
Question 7.
Write the dispersed phase and dispersion medium of the following colloidal systems:
(i) Smoke
(ii) Milk
OR
What are lyophilic and lyophobic colloids? Which of these sols can be easily coagulated on the addition of small amounts of electrolytes? (CBSE Delhi 2013)
Answer:
| Colloidol System | Dispersed phase | Dispersed medium |
| (i) Smoke | Solid | Gas |
| (ii) Milk | Liquid | Liquid |
OR
The colloidal solutions in which the particles of the dispersed phase have great affinity for the dispersion medium are called lyophilic sols. For example, glue, gelatin, starch, proteins, etc.
The colloidal solutions in which there is no affinity between the particles of the dispersed phase and the dispersion medium are called lyophobic sols. For example, ‘ solutions of metals like Ag, Au, Al(OH)3, Fe(OH)3, etc.
Lyophobic sols can be easily coagulated on the addition of small amounts of electrolytes.
Question 8.
What are emulsions? What are their different types? Give one example of each type. (CBSE 2014)
Answer:
Emulsions are the colloidal solutions of two immiscible liquids in which the liquid acts as the dispersed phase as well as the dispersion medium.
Types of emulsions. These are of two types:
1. Oil-in-water emulsion. In this case, oil acts as the dispersed phase (small amount) and water as the dispersion medium (excess), e.g. milk is an emulsion of soluble fats in water and here casein acts as an emulsifier. Vanishing cream is another example of this class. Such emulsions are called aqueous emulsions.
(ii) Water-in-oil emulsion. In this case, water acts as the dispersed phase while the oil behaves as the dispersion medium, e.g. butter, cod liver oil, cold cream, etc. Such types of emulsions are called oily emulsions.
Question 9.
(i) In reference to Freundlich adsorption isotherm, write the expression for adsorption of gases on solids in the form of an equation.
(ii) Write an important characteristic of lyophilic sols.
(iff) Based on type of particles of dispersed phase, give one example each of associated colloid and multimolecular colloid. (CBSE Delhi 2014)
Answer:
(i) \(\frac{x}{m}\) = kp1/n
where \(\frac{x}{m}\) is extent of adsorption, x is the mass of adsorbate, m is the mass of adsorbent, p is pressure at a particular temperature, k is a constant and n takes any whole number value.
(ii) in lyophilic sols, the particles of dispersed phase have a great affinity for the dispersion medium and are reversible in nature.
(iii) Associated colloids: Soap (e.g. sodium stearate).
Multimolecular colloids: Sol of gold (atoms).
Question 10.
Give reasons for the following observations:
(i) Leather gets hardened after tanning.
(ii) Lyophilic sol is more stable than lyophobic sol.
(iii) It is necessary to remove CO when ammonia is prepared by Haber’s process. (CBSE Delhi 2015)
Answer:
(i) Animal hide is colloidal in nature and has positively charged particles. When it is soaked in tannin which has negatively charged colloidal particles, it results in mutual coagulation. This results in the hardening of leather.
(ii) Lyophilic sol is more stable than lyophobic sol because it is highly hydrated or solvated in solution.
(iii) Carbon monoxide acts as a poison for the catalyst in Haber’s process and therefore, it will lower the activity of the catalyst.
Thus, CO must be removed when ammonia is obtained by Haber’s process.
Question 11.
Give reasons for the following observations:
(i) Physisorption decreases with increase in temperature.
(ii) Addition of alum purifies the water.
(iii) Brownian movement provides stability to the colloidal solution. (CBSE 2015)
Answer:
(i) In physisorption, the attractive forces between adsorbent and adsorbate molecules are weak vander Waals forces. When temperature is increased, the kinetic energy of the molecules of the gas increases and they can easily leave the surface of adsorbent.
Therefore, physisorption decreases with increase in temperature.
(ii) Alum coagulates the impurities present in water by neutralising the charge.
(iii) The Brownian movement is due to the unbalanced bombardment of the particles by the molecules of the dispersion medium. This results in zigzag motion. Because of brownian movement, the particles do not settle down and hence is responsible for the stability of the sols.
Question 12.
Define the following terms:
(i) O/W Emulsion
(ii) Zeta potential
(iii) Multimolecular colloids (CBSE 2016)
Answer:
(i) O/W Emulsion: It is the emulsion in which oil is the dispersed phase and water is the dispersion medium. For example, milk is an emulsion of soluble fats in water.
(ii) Zeta potential: The potential difference between the fixed layer and the diffused layer of opposite charges is called Zeta potential.
(iii) Multimolecular colloids: When a dissolution, atoms or smaller molecules of substances (having diameter less than 1 nm) aggregate together to form particles of colloidal dimension, the particles thus formed are called multimolecular colloids.
Question 13.
(i) Differentiate between adsorption and absorption.
(ii) Out of MgCl2 and AlCl2, which one is more effective in causing coagulation of negatively charged sol and why?
(iii) Out of sulphur sol and proteins, which one forms multimolecular colloids? (CBSE Delhi 2016)
Answer:
(i)
| Absorption | Adsorption |
| 1. It is the phenomenon in which the particles of gas or liquid get uniformly distributed throughout the body of the solid. | 1. It is the phenomenon of higher concentration of particles of gas or liquid on the surface than in the bulk of the solid. |
| 2. The concentration is the same throughout the material. Therefore, it is a bulk phenomenon. | 2. The concentration on the surface of the adsorbent is different from that in the bulk. Therefore, it is a surface phenomenon. |
| 3. Absorption occurs at uniform rate. | 3. Adsorption is rapid in the beginning and its rate slowly decreases. |
(ii) According to Hardy-Schulze rule, for negatively charged sol, greater the valency of the positive ion of the electrolyte added, greater is its coagulating power. Thus AlCl3 (Al3+ ion) is more effective in causing coagulation of negatively charged sol than MgCl2 (Mg2+ ions).
(iii) Proteins are macromolecules which cannot form multimolecular colloids while sulphur sol has smaller S8 molecules which can coagulate to form multimolecular colloids.
Question 14.
What is meant by coagulation of a colloidal solution? Describe briefly any three methods by which coagulation of lyophobic sols can be carried out.
(CBSE Delhi 2013)
Answer:
The phenomenon of precipitation of a colloidal sol by the addition of excess of an electrolyte is called coagulation of colloidal sol.
Coagulation of lyophobic sols can be carried out by the following methods:
(i) By addition of an electrolyte: When an electrolyte is added to the sol, colloidal particles take up ions carrying opposite charge from the electrolyte and get neutralised resulting in coagulation.
(ii) By mutual precipitation: When two oppositely charged sols are mixed in equimolar proportions, they mutually neutralise their charge and both get coagulated.
(iii) By electrophoresis: When electrophoresis is carried out for a long time, the particles of dispersed phase move towards oppositely charged electrodes, lose their charge and get coagulated.
Question 15.
(i) What is the role of activated charcoal in gas mask?
(ii) A colloidal sol is prepared by the given method in figure. What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?
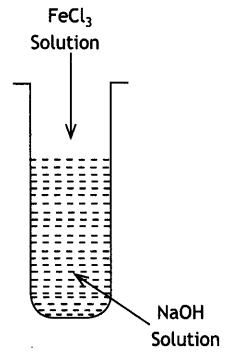
(iii) How does chemisorption vary with temperature? (CBSE Delhi 2019) Answer:
(i) Activated charcoalin gas mask adsorbs the poisonous or toxic gases from air. Therefore, these masks help to purify the air for breathing.
(ii) Negatively charged sol
Representation: Fe2O3. xH2O/OH–
(iii) In chemisorption, adsorption initially increases with rise in temperature and then decreases.
Question 16.
Define the following terms with an example in each case:
(i) Macromolecular sol
(ii) Peptization
(iii) Emulsion (CBSE Al 2013)
Answer:
(i) Macromolecular sol: In this type, the particles of the dispersed phase are sufficiently big in size (macro) to be of colloidal dimensions. In this case, a large number of small molecules are joined together through their primary valencies to form giant molecules. These molecules are called macromolecules and each macromolecule may consist of hundreds or thousands of simple molecules. The solutions of such molecules are called macromolecular solutions. For example, colloidal solution of starch, cellulose, etc.
(ii) Peptization: The process of converting a freshly prepared precipitate into colloidal form by addition of a suitable electrolyte is called peptization. The electrolytes used for the purpose are called peptizing agents.
(iii) Emulsions: They are the colloidal solutions of two immiscible liquids in which the liquid acts as the dispersed phase as well as the dispersion medium. Normally, these are obtained by mixing an oil with water. Since the two do not mix well, the emulsion is generally unstable and is stabilised by adding a suitable reagent called emulsifier or emulsifying agent. The substances that are commonly employed for the purpose are gum, soap, glass powder, etc.
Question 17.
Define the following with a suitable example of each:
(a) Coagulation
(b) Multimolecular colloid
(c) Gel
Answer:
(a) Coagulation. The phenomenon of precipitation of a colloidal solution by the addition of excess of an . electrolyte is called coagulation. For
example, Fe(OH)3 colloidal solution is coagulated by adding HCl.
(b) Multimolecular colloid. When on dilution, atoms or smaller molecules of substances (having diameter less than 1 nm) aggregate together to form particles of colloidal dimensions, then the particles thus formed are called multimolecular colloids, for example, sols of gold atoms.
(c) Gel. A gel is a colloidal system in which a liquid is dispersed in a solid. For example, jellies, cheese.
OR
(a) Out of starch and ferric hydroxide sol, which one can easily be coagulated and why?
(b) What is observed when an emulsion is centrifuged?
(c) What is the role of promoters and poisons in catalysis? (CBSE Al 2019)
Answer:
(a) Ferric hydroxide sol is easier to coagulate because it is a lyophobic sol.
(b) De-emulsification occurs.
(c) Promoters increase the efficiency of catalyst whereas poisons inhibit the efficiency of catalyst.
Question 18.
Give reason for the following observations:
(i) When silver nitrate solution is added to potassium iodide solution, a negatively charged colloidal solution is formed.
(ii) Finely divided substance is more effective as an adsorbent.
(iii) Lyophilic colloids are also called reversible sols.
Answer:
(i) The precipitated silver iodide adsorbs iodide ions from the dispersion medium resulting in the negatively charged colloidal solution.
(ii) Due to large surface area
(iii) If the dispersion medium is separated from the dispersed phase, the sol can be reconstituted by simply remixing with the dispersion medium. That is why these sols are also called reversible sols.
Question 19.
Answer the following questions:
(a) Which of the following electrolytes is most effective for the coagulation of Agl/Ag+ sol?
MgCl2, K2SO4, K4[Fe(CN)6]
(b) What happens when a freshly precipitated Fe(OH)3 is shaken with a little amount of dilute solution of FeCl3?
(c) Out of sulphur sol and proteins, which one forms macromolecular colloids? (CBSE Sample Paper 2019)
Answer:
(a) K4[Fe(CN)6]
Hardy Schulze rule: The coagulation tendency of different electrolytes is different. It depends upon the valency of the active ion called flocculatins ion, which is the ion-carrying charge opposite to the charge on the colloidal particles. According to Hardy Schulze rule, greater the valency of the active ion or flocculating ion, greater will be its coagulating power. For example, to coagulate negative sol of AS2S3, the coagulating power of different cations has been found to decrease in the order as:
Al3+ > Mg2+ > Na+
Similarly, to coagulate a positive sol such as Fe(OH)3, the coagulating power of different anions has been found to decrease in the order:
[Fe(CN)6]4– > PO43- > SO42- > Cl–
(b) Due to the phenomenon of peptization, a reddish brown positively charged sol of ferric hydroxide is obtained when a freshly precipitated Fe(OH)3 is shaken with a little amount of dilute solution of FeCl3. It involves the adsorption of suitable ions from the electrolyte by . the particles of the precipitate. The charged particles repel one another and form a colloidal sol. Ferric ions from ferric chloride are adsorbed by Fe(OH)3, precipitate and get converted into colloidal sol.
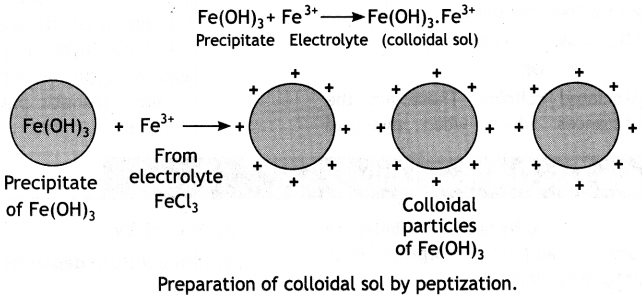
(c) Proteins are macromolecules which cannot form multi-molecular colloids while sulphur sol has smaller S8 molecules which can coagulate to form multi-molecular colloids.
Question 20.
Answer the following:
(a) Why is ester hydrolysis slow in the beginning and then becomes faster after some time?
(b) In a solution of methylene blue, animal charcoal is added, the solution is then well shaken. What will be observed and why?
(c) Give an example of oil in water emulsion.
Answer:
(a) This is because of the process of autocatalysis. Ester on hydrolysis gives an acid which starts acting as a catalyst after sometime and therefore, the reaction becomes fast.
(b) Adsorption of a dye by charcoal. When animal charcoal is shaken with a solution of an organic dye such as methylene blue it is observed that the solution turns colourless. The discharge of the colour is due to the fact that the coloured component (generally an organic dye) gets adsorbed on the surface of animal charcoal. Therefore, animal charcoal is used for decolourising a number of organic substances in the form of their solutions.
(c) Milk, vanishing cream.
OR
Define the following:
(a) Associated colloids
(b) Electrophoresis
(c) Zeta potential (CBSE 2019C)
Answer:
(a) Associated colloids: These are the substances which when dissolved
in a medium behave as normal electrolytes at low concentration but behave as colloidal particles at higher concentration due to the formation of aggregated particles. The aggregate particles thus formed are called micelles. For example, in aqueous solution, soap (sodium stearate) ionises as:
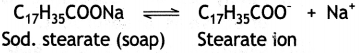
In concentrated solution, these ions get associated to form an aggregate of colloidal size.
(b) The phenomenon of movement of colloidal particles under an applied electric field is called electrophoresis. If the particles accumulate near the negative electrode, the charge on the particles is positive. On the other hand, if the sol particles accumulate near the positive electrode, the charge on the particles is negative.
(c) The combination of two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer. The first layer of ions is firmly held and is termed fixed layer while the second layer is mobile which is termed diffused layer. The charges of opposite signs on the fixed and diffused parts of the double layer result in a difference in potential between these layers. The potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.
Question 21.
Write the differences between physisorption and chemisorption With respect to the following:
(i) Specificity
(ii) Temperature dependence
(iii) Reversibility and
(iv) Enthalpy change (CBSE Delhi 2013)
Answer:
| Property | Physisorption | Chemisorption |
| (i) Specificity: | not specific | highly specific |
| (ii) Temperature dependence: | Occurs at low temperature and decreases with increasing temperature | Occurs at high temperature and increases with increasing temperature |
| (iii) Reversibility: | Reversible in nature | Irreversible in nature |
| (iv) Enthalpy change: | Low enthalpy of adsorption (20 – 40 kJ mol-1) | High enthalpy of adsorption (80 – 240 kJ mol-1) |
Question 22.
Describe some features of catalysis by zeolites.
Answer:
Zeolites are microporous aluminosilicates of the general formula Mx/n [(AlO2)x (SiO2)y] mH2O. These are most important oxide catalysts. These are used in petrochemical industries for cracking of hydrocarbons and isomerisation. The reactions in zeolites depend upon the size of the cavities (cages) or pores (apertures) present in them.
The most remarkable feature of zeolite catalysis is the shape selectivity. Therefore, the selectivity of catalyst depends on the pores structure. It has been observed that the pore size in zeolites generally varies between 260 pm and 740 pm. Depending upon the size of the reactants and products compared to the size of the cages or pores of zeolite, reactions proceed in specific manner.
A zeolite catalyst called ZSM-5 converts alcohols to gasoline, by first dehydrating the alcohol by loss of water.
Question 23.
What do you understand by adsorption isotherm? Discuss briefly Freundlich adsorption isotherm.
Answer:
Adsorption isotherm. A graph between amount of adsorption and gas pressure keeping temperature constant is called an adsorption isotherm.
Freundlich adsorption isotherm. The behaviour of adsorption with pressure can be expressed by adsorption isotherm as shown in figure.
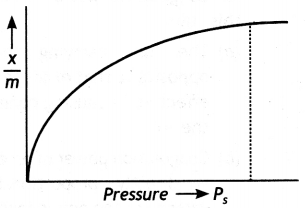
The inspection of the graph reveals the following facts:
(i) At low pressure, the graph is almost straight line which indicates that x/m is directly proportional to the pressure. This may be expressed as:
\(\frac{x}{m}\) ∝ P or \(\frac{x}{m}\) = kP ……. (i)
where k is constant.
(ii) At high pressure. The graph becomes almost constant which means that x/m becomes independent of pressure. This may be expressed as:
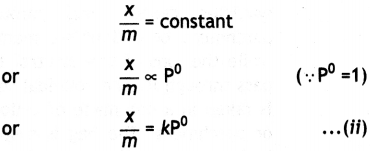
(iii) Thus, in the intermediate range of pressure, x/m will depend upon the power of pressure which lies between 0 and 1, i.e. fractional power of pressure. This may be expressed as:
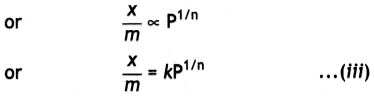
where n can take any value between 1 and a larger integer depending upon the pressure. The above relationship is also called Freundlich’s adsorption isotherm.
Question 24.
Explain the following in connection with colloids:
(i) Hardy-Schulze rule
(ii) Dialysis.
Answer:
(i) Hardy-Schulze rule: This rule states that greater the valency of the active ion or flocculating ion, greater will be its coagulating power. According to this rule:
(a) The ions carrying the charge opposite to that of sol particles are effective in causing coagulation of the sol.
(b) Coagulation power of an electrolyte is directly proportional to the valency of the active ions.
For example, for negative As2S3 sol, the coagulating power of cations decreases as Al3+ > Mg2+ > K+
Similarly, for positively charged sol such as Fe (OH)3, the coagulating power decreases as
[Fe(CN)3]4- > PO43- > SO42- > Cr–
(ii) Dialysis. The method used to separate the impurities from the colloidal solution is called dialysis. Its principle is based upon the fact that colloidal particles cannot pass through a parchment or cellophane membrane while the ions of the electrolyte can pass through it. The colloidal solution is taken in a bag made of cellophane or parchment. The bag is suspended in fresh water. The impurities slowly diffuse out of the bag leaving behind pure colloidal solution. Dialysis can be used for removing HCl from the ferric hydroxide sol. The method is shown in figure.
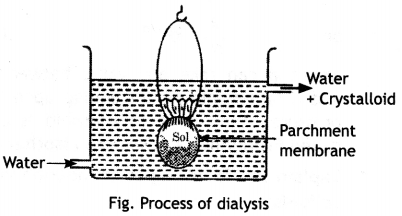
The ordinary process of dialysis is slow. To increase the process of purification, the dialysis is carried out by applying electric field. This process is called electrodialysis.
Question 25.
Explain the following terms:
(i) Electrodialysis
(ii) Tyndall effect.
Answer:
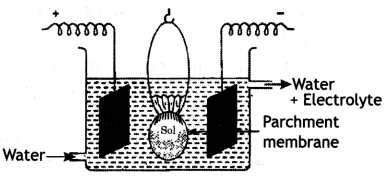
(i) Electrodialysis. It is the process of separating the impurities from the colloidal solution by applying electric field. It is based on the fact that colloidal particles cannot pass through a parchment or cellophane membrane while the ions of the electrolyte can pass through it. The colloidal solution is taken in a bag made of cellophane or parchment and is suspended in a fresh water and electric current is applied. The impurities slowly diffuse out of the bag leaving behind pure colloidal solution.
(ii) Tyndall effect. When a strong beam of light is passed through a true solution placed in a beaker, in a dark room, the path of the light does not become visible. However, if the light is passed through a sol, placed in the same room, the path of the light becomes visible when viewed from a direction at right angle to that of the incident beam.
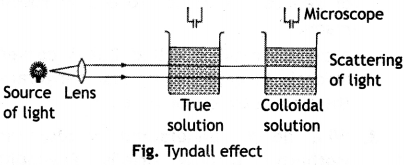
This phenomenon was studied for the first time by Tyndall and therefore it is called Tyndall effect. The cause to Tyndall effect is the scattering of light by colloidal particles, i.e. these particles first absorb the incident light and then a part of it gets scattered by them. Since the intensity of the scattered light is at right angle to the plane of the incident light, the path becomes visible only when seen in that direction. The particles in true solution are too small in size to cause any scattering, i.e. the Tyndall effect is not noticed in true solutions.