Here we are providing Class 12 Chemistry Important Extra Questions and Answers Chapter 6 General Principles and Processes of Isolation of Elements. Class 12 Chemistry Important Questions are the best resource for students which helps in Class 12 board exams.
Class 12 Chemistry Chapter 6 Important Extra Questions General Principles and Processes of Isolation of Elements
General Principles and Processes of Isolation of Elements Important Extra Questions Very Short Answer Type
Question 1.
What is the composition of copper matte? (CBSE Delhi 2013)
Answer:
Copper matte contains cuprous sulphide (Cu2S) and iron sulphide (FeS).
Question 2.
Name the method used for refining of
(i) Nickel
(ii) Zirconium (CBSE Sample Paper 2007, 2014)
Answer:
(i) Mond’s process
(ii) Van Arkel method
Question 3.
Write the overall reaction taking place in the process used for the electrolysis of alumina by Hall-Heroult process. (CBSE Sample Paper 2011)
Answer:
2Al2O3 + 3C → 4Al + 3CO2
Question 4.
Write a non-exothermic reaction taking place in the blast furnace during extraction of iron. (CBSE Sample Paper 2011)
Answer:
![]()
Question 5.
Name the method of refining of metals such as germanium. (CBSE Delhi 2016)
Answer:
Zone refining.
Question 6.
In the extraction of Al, impure Al2O3 is dissolved in cone. NaOH to form sodium aluminate and leaving impurities behind. What is the name of the process? (CBSE Delhi 2016)
Answer:
Al2O3 + 2NaOH + 3H2O → 2Na [Al(OH)4]
This process is called leaching.
Question 7.
Name the method of refining which is based on the principle of adsorption.
(CBSE AI 2016)
Answer:
Chromatography.
Question 8.
Out of PbS and PbCO3 (ores of lead), which one is concentrated by froth floatation process preferably? (CBSE Delhi 2017)
Answer:
PbS
Question 9.
What is the role of depressants in the froth floatation process? (CBSE Al 2017)
Answer:
The depressants in the froth floatation process selectively prevent one of the sulphide ores from forming the froth with air bubbles.
Question 10.
What is the role of collector and froth stabilizer in froth floatation process?
(CBSE AI 2012)
Answer:
- Collector enhances non-wettability of the mineral particles.
- Froth stabilisers stabilise the froth.
Question 11.
Name the method used for refining of copper. (CBSE AI 2013)
Answer:
Electrolytic refining
Question 12.
What is the role of graphite in the electrometallurgy of aluminium? (CBSE Delhi 2012)
Answer:
The graphite rod is useful in the electrometallurgy of aluminium for reduction of alumina to aluminium.
2Al2O3 + 3C → 4Al + 3CO2
Question 13.
Why is it that only sulphide ores are concentrated by ‘froth floatation process’? (CBSE Delhi 2011)
Answer:
This is because the sulphide ore particles are preferentially wetted by oil and the gangue particles by water.
Question 14.
What is the role of collectors in Froth- Floatation process? (CBSE AI 2012, 2017)
Answer:
The collectors such as pine oil, eucalyptus oil and fatty acids enhance the non – wettability of the mineral particles in froth – floatation process.
Question 15.
Name the substance used as depressant in the separation of two sulphide ores in froth floatation method. (CBSE Sample Paper 2019)
Answer:
Sodium cyanide.
Question 16.
Which reducing agent is employed to get copper from the leached low grade copper ore? (CBSE Delhi 2014)
Answer:
Iron scraps.
Question 17.
What is the role of zinc metal in the extraction of silver? (CBSE 2014)
Answer:
Zinc acts as a reducing agent.
General Principles and Processes of Isolation of Elements Important Extra Questions Short Answer Type
Question 1.
Out of C and CO which is a better reducing agent for FeO?
(i) In the lower part of blast furnace (Higher temperature)
(ii) In the upper part of blast furnace (Lower temperature) (CBSE Sample Paper 2011)
Answer:
(i) C is better reducing agent at higher temperature (lower part of blast furnace).
(ii) CO is better reducing agent at lower temperature (higher part of blast furnace).
Question 2.
What is the role of limestone in the extraction of iron from its oxides? (CBSE Al 2016)
Answer:
Limestone decomposes to form lime (CaO) and carbon dioxide (CO2). The lime thus produced acts as a flux and combines with silica (present as impurity in oxides of iron) to produce slag.
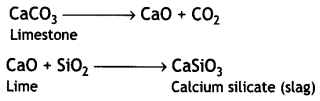
Question 3.
How is copper extracted from a low grade ore of it? (CBSE Al 2012)
Answer:
Copper is extracted by hydrometallurgy from low grade ores. It is leached out using acid or bacteria. The solution containing copper ions (Cu2+) is treated with scrap iron or H2 as:
Cu2+(aq) + H2(g) → Cu(s) + 2H+(aq)
In this way, copper is obtained.
Question 4.
(i) Which solution is used for the leaching of silver metal in the presence of air in the metallurgy of silver?
(ii) Out of C and CO, which is a better reducing agent at the lower temperature range in the blast furnace to extract iron from the oxide ore? (CBSE Delhi 2013)
Answer:
(i) Dilute solution (0.5%) of NaCN or KCN in the presence of air.
(ii) CO
Question 5.
(i) Which of the following ores can be concentrated by froth floatation method and why?
Fe2O3, ZnS, Al2O3
(ii) What is the role of silica in the metallurgy of copper? (CBSE Delhi 2013)
Answer:
(i) ZnS, because in sulphide ores, the sulphide ore particles are preferentially wetted by oil and gangue particles by water.
(ii) During roasting, the copper pyrites are converted into a mixture of FeO and Cu2O.
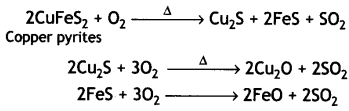
To remove FeO (basic), the roasted ore is mixed with silica and heated. Silica acts as a flux and combines with ferrous oxide present to form fusible slag of iron silicate.
![]()
The slag being lighter floats and forms the upper layer and is removed through slag hole. Therefore, silica helps to remove FeO in the metallurgy of copper.
Question 6.
(i) Name the method used for removing gangue from sulphide ores.
(ii) How is wrought iron different from steel? (CBSE Al 2013)
Answer:
(i) Froth floatation method.
(ii) Wrought iron is the pure form of iron and contains carbon and other impurities not more than 0.5%.
Steel contains 0.5 to 1.5% carbon along with small amounts of other elements such as Mn, Cr, Ni, etc. and other impurities.
Question 7.
Outline the principles behind the refining of metals by the following methods: (CBSE Delhi 2014)
(i) Zone refining method
(ii) Chromatographic method
Answer:
(i) Zone refining method. It is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
(ii) Chromatographic method. This is based on the principle that different components of a mixture are differently adsorbed on an adsorbent.
Question 8.
Write the principle behind the froth floatation process. What is the role of collectors in this process? (CBSE 2014)
Answer:
The froth floatation process is based on the principle of difference in the wetting properties of the ore and gangue particles with water and oil. The collectors such as pine oil, eucalyptus oil, fatty acids, etc. are used to enhance the non-wettability of the mineral particles.
Question 9.
What is meant by vapour phase refining? Write any one example of the process which illustrates this technique, giving the chemical equations involved.
OR
Write and explain the reactions involved in the extraction of gold. (CBSE Sample Paper 2019)
Answer:
Vapour phase refining: This method is based on the fact that certain metals are converted to their volatile compounds while the impurities are not affected during compound formation. The compound formed decomposes on heating to give pure metal. Thus, the two requirements are:
- The metal should form a suitable compound with a suitable reagent.
- The volatile compound should be easily decomposable so that the metal can be easily recovered.
For example, nickel is refined by this technique and the method is known as Mond process. In this method, nickel is heated in a stream of carbon monoxide to form volatile nickel tetracarbonyl, Ni(CO)4, complex.
The carbonyl vapours when subjected to higher temperature (450-470 K) undergo thermal decomposition giving pure nickel.
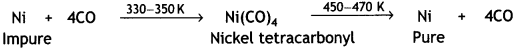
OR
The extraction of gold involves leaching of metal present in the ore with CN– ions. This is an oxidation-reaction because during the leaching process, Au is oxidised to Au+ which then combines with CN– ions to form their respective soluble complexes.
![]()
The metal is then recovered from these complexes by reduction or displacement method using a more electropositive zinc metal.
2 [Au (CN)2]– (aq) + Zn(s) → 2 Au (s) + [Zn (CN)4]2- (aq)
Zinc acts as a reducing agent.
This process is called hydro metallurgy.
General Principles and Processes of Isolation of Elements Important Extra Questions Long Answer Type
Question 1.
(i) Give one example of each the following:
(a) Acidic flux
(b) Basic flux
(ii) What happens when
(a) Cu2O undergoes self reduction in a silica line converter
(b) Haematite oxidises carbon to carbon monoxide? (CBSE Sample Paper 2012)
Answer:
(i) (a) Acidic flux: SiO2
(b) Basic flux: CaO
(ii) (a) Cu2O undergoes self reduction to form blister copper as:
![]()
(b) Haematite oxidises carbon to carbon monoxide forming iron.
Fe2O3 + 3C → 3CO + 2Fe
Question 2.
What is a flux? What is the role of flux in the metallurgy of iron and copper? (CBSE Sample Paper 2011)
Answer:
Flux is a substance which combines with gangue which may still be present in the calcined or roasted ore to form an easily fusible material called the slag.
Flux + Gangue → Slag (fusible)
In the metallurgy of copper, most of the ferrous sulphide present as impurity gets oxidised to ferrous oxide which combines with silica (flux) to form fusible slag.
FeO + SiO2 → FeSiO3
The slag being lighter floats and forms the upper layer which is removed from the slag hole from time to time.
In the blast furnace for metallurgy of iron, lime acts as a flux and combines with silica present as impurity to form slag.
![]()
Question 3.
Describe the principle involved in each of the following processes.
(i) Mond process for refining of Nickel.
(ii) Column chromatography for purification of rare elements. (CBSE 2012)
Answer:
(i) Mond’s process for refining of nickel. Impure nickel is heated in a stream of carbon monoxide forming volatile complex, tetra carbonylnickel (0).
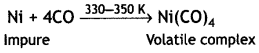
The volatile complex is decomposed at high temperature to give pure metal
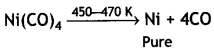
(ii) Column chromatography for purification of rare elements. This technique is based on the differences in the adsorbing capacities of the metal and its impurities. The metal and the impurities are differently adsorbed. Impure metal is put in a liquid or gaseous medium (called moving phase) which is moved through an adsorbent (stationary phase).
Metal and impurities are adsorbed at different levels in column.
Question 4.
Which methods are usually employed for purifying the following metals?
(i) Nickel
(ii) Germanium
Mention the principle behind each one of them. (CBSE 2012)
Answer:
(i) Nickel. By vapour phase refining. This method is based on the principle that certain metals are converted to their volatile compounds while the impurities are not affected during compound formation. The compound formed decomposes on heating to give pure metal.
For example, nickel is refined by this technique and the method is known as Mond process. In this method, nickel is heated in a steam of carbon monoxide to form volatile nickel carbonyl Ni(CO)4.
The carbonyl vapours when subjected to higher temperature (450-470 K) undergo thermal decomposition giving pure nickel.
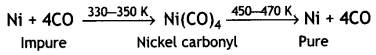
(ii) Germanium. By Zone refining method. This method is based on the principle that the impurities are more soluble in melt than in the solid state of the metal. Therefore, an impure metal on solidification will deposit pure metal and the impurities will remain behind in the molten part of the metal.
Question 5.
Explain the principle of the method of electrolytic refining of metals. Give one example. (CBSE 2014)
Answer:
In electrolytic refining method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode. Both anode and cathode are placed in a suitable electrolytic bath containing soluble salt of the same metal. On passing the electrical current, metal ions from the electrolyte are deposited at the cathode in the form of pure metal, while equivalent amount of metal dissolves from the anode into the elctrolyte in the form of metal ions. The impurities fall down below the anode as anode mud. The reactions occurring at the electrodes are:
At cathode: Mn+ + ne– → M
At anode: M → Mn+ + ne–
Copper is refined using electrolytic refining method.
Question 6.
(i) Name the method used for the refining of zirconium. (CBSE 2015)
(ii) What is the role of CO in the extraction of iron?
(iii) Reduction of metal oxide to metal becomes easier if the metal obtained is in liquid state. Why?
Answer:
(i) Van Arkel method
(ii) CO acts as reducing agent. It reduces oxides of iron to iron.
FeO + CO → Fe + CO2
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
(iii) This is because if the metal is in liquid state, its entropy is higher than when it is in solid state. Therefore, the value of entropy change (ΔS) of the reduction process is more on +ve side when the metal formed is in the liquid state and the metal oxide being reduced is in solid state. As a result, the value of ΔG° becomes more on negative side and hence the reduction becomes easier.
Question 7.
(i) Name the method of refining which is based on the principle of adsorption. (CBSE 2008, 2016)
(ii) What is the role of depressant in froth floatation process?
(iii) What is the role of limestone in the extraction of iron from its oxides?
Answer:
(i) Chromatography
(ii) The depressants are used to prevent certain types of particles from forming the froth with bubbles in froth floatation process. This helps to separate two sulphide ores. For example, in case of an ore containing zinc sulphide (ZnS) and lead sulphide (PbS), sodium cyanide (NaCN) is used as a depressant. It forms a layer of zinc complex Na2[Zn(CN)4] with ZnS on the surface of ZnS and therefore, prevents it from forming the froth. Therefore, it acts as a depressant.
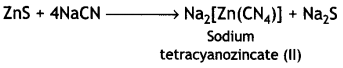
However, NaCN does not prevent PbS from forming the froth and allows it to come with the froth.
(iii) Limestone decomposes to form lime (CaO) and carbon dioxide (CO2). The lime thus produced acts as a flux and combines with the silica (present as impurity in oxides of iron) to produce slag.
Question 8.
(i) Name the method of refining of metals such as germanium. (CBSE Delhi 2016)
(ii) In the extraction of Al, impure Al2O3 is dissolved in cone. NaOH to form sodium aluminate and leaving impurities behind. What is the name of this process?
(ii) What is the role of coke in the extraction of iron from its oxides?
Answer:
(i) Zone refining
(ii) Al2O3 + 2NaOH + 3H2O → 2Na[Al(OH)4]
This process is called leaching.
(iii) The coke serves as a fuel as well as a reducing agent. It burns to produce CO2 and heat which raises the temperature to about 2200 K (in combustion zone).
C + O2 → CO2, ΔH = -393.4 kJ
It also reduces Fe2O3 to iron.
Fe2O3 + 3C → 2Fe + 3CO + Heat
Question 9.
(i) Indicate the principle behind the method used for the refining of zinc.
(ii) What is the role of silica in the extraction of copper?
(iii) Which form of the iron is the purest form of commercial iron? (CBSE Delhi 2015)
Answer:
(i) Zinc is refined by electrolytic refining:
In this method, the impure zinc is made anode, while a plate of pure zinc is made the cathode. These are placed in a suitable electrolytic bath containing suitable salt of the same metal (e.g. zinc sulphate) containing a small amount of dilute sulphuric acid. On passing current, zinc from the solution is deposited at the cathode, while an equivalent amount of zinc from anode goes into the electrolytic solution. Therefore, pure zinc is obtained at cathode.
(ii) During roasting, the copper pyrites are converted into a mixture of FeO and Cu2O.
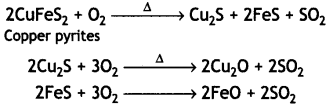
To remove FeO (basic), the roasted ore is mixed with silica and heated. Silica acts as a flux and combines with ferrous oxide present to form fusible slag of iron silicate.
![]()
The slag being lighter floats and forms the upper layer and is removed through slag hole. Therefore, silica helps to remove FeO in the metallurgy of copper.
(iii) Wrought iron is the purest form of commercial iron.
Question 10.
(a) What is the difference between calamine and malachite?
(b) Why is zinc used instead of Cu for recovery of Ag from [Ag(CN)2]?
(c) What is the role of cryolite in metallurgy of Al? (CBSE 2019C)
OR
(a) Give two points of differences between pig iron and cast iron.
(b) Outline the principle of zone refining.
Answer:
(a) Calamine is an ore of Zn while malachite is an ore of copper. Calamine is ZnCO3 while malachite is CuCO3.Cu(OH)2
(b) Zinc is more electropositive than silver and therefore, zinc displaces silver from its solution.
2[Ag(CN)2]– + Zn → [Zn(CN)4]2- + 2Ag
On the other hand, copper is less electropositive than silver and therefore, cannot displace silver from its solution. Zn is more reactive than Cu, so reduction will be faster in case of Zn.
(c) Fused alumina is a bad conductor of electricity. Purified alumina is mixed with molten cryolite (Na3AlF6) and is electrolysed in an iron tank lined inside with carbon. The molten cryolite decreases the melting point and increases the electrical conductivity. It acts as a solvent.
OR
(a)
| Cast Iron | Pig Iron |
| 1. It is moulded pig iron. | 1. It is obtained directly from the blast furnace. |
| 2. It is made by melting pig iron with scrap iron and coke using hot air blast. | 2. It dissolves some sulphur, silicon, phosphorus and manganese as impurities. |
| 3. It contains about 3% carbon. | 3. It contains about 4% carbon. |
| 4. It is extremely hard and brittle. | 4. It is more brittle. |
(b) Zone refining – It is based on the principle that impurities are more soluble in the melt than the solid state of the metal. Therefore, an impure metal on solidification will deposit crystals of pure metal and the impurities will remain behind in the molten part of the metal.
Question 11.
How will you convert the following:
(i) Impure Nickel to pure Nickel
(ii) Zinc blende to Zinc metal
(iii) [Ag(CN)2]– to Ag (CBSE Delhi 2019)
Answer:
(i) Impure nickel is heated in a stream of carbon monoxide forming volatile complex tetracarbonylnickel (0). The volatile complex is decomposed at high tempreature to give pure nickel.

This is called Mond’s process.
(ii) The zinc blende ore is concentrated by froth floatation process and then roasted in the presence of excess air at about 1200 K.
2ZnS + 3O2 → 2ZnO + 2SO2
Zinc oxide formed is reduced to zinc by heating with crushed coke at 1673 K in vertical fire clay retorts.

(iii) [Ag(CN)2]– complex is heated with zinc to get silver.
2[Ag(CN)2]– + Zn → [Zn(CN)4]2- + 2Ag
Question 12.
(a) Name the method of refining which is
(i) used to obtain semiconductor of high purity,
(ii) used to obtain low boiling metal.
(b) Write chemical reactions taking place in the extraction of copper from Cu2S. (CBSE Delhi 2019)
Answer:
(a) (i) Zone refining
(ii) Distillation
(b) Cu2S is first roasted and converted to oxide
2Cu2S + 3O2 → 2Cu2O + 2SO2
The oxide is then easily reduced to metallic copper with coke.
Cu2O + C → 2Cu + CO
Question 13.
Describe how the following steps can be carried out.
(i) Recovery of Gold from leached gold metal complex.
(ii) Conversion of Zirconium iodide to pure Zirconium.
(iii) Formation of slag in the extraction of copper.
(Write the chemical equations also for the reactions involved)
OR
Explain the use of the following:
(i) NaCN in Froth Floatation Method.
(ii) Carbon monoxide in Mond process.
(iii) Coke in the extraction of Zinc from Zinc Oxide.
Answer:
(i) Leached gold complex is treated with Zinc and gold is recovered by displacement method.
2Au[(CN)2]r(aq) + Zn(s) → 2Au(s) + [Zn(CN)4]2-(aq)
(ii) Zirconium iodide is decomposed on a tungsten filament, electrically heated to 1800 K. Pure Zr metal is deposited on the filament.
ZrI4 → Zr + I2
(iii) Silica is added to the ore and heated. It helps to slag off iron oxide as iron silicate.
FeO + SiO2 → FeSiO3 (slag)
OR
(i) NaCN is used as depressants to separate two sulphide ores (ZnS and PbS) in Froth Floatation Method.
(ii) Carbon monoxide forms a volatile complex of nickel, nickel tetracarbonyl.
(iii) Coke is used as a reducing agent to reduce zinc oxide to zinc.
Question 14.
Write the role of
(i) NaCN in the extraction of gold from its ore.
(ii) Cryolite in the extraction of aluminium from pure alumina.
(iii) CO in the purification of Nickel. (CBSE 2018)
Answer:
(i) Gold is leached out in the form of a complex with dil. solution of NaCN in the presence of air. Here NaCN acts as leaching agent.
(ii) It lowers the melting point of alumina and makes it a good conductor of electricity.
(iii) CO forms a volatile complex with nickel which is further decomposed to give pure Ni metal.
Question 15.
(i) Write the role of ‘CO’ in the purification of nickel.
(ii) What is the role of silica in the extraction of copper?
(iii) What type of metals are generally extracted by electrolytic method? (CBSE Delhi 2019)
Answer:
(i) Carbon monoxide forms a volatile complex, Ni(CO)4, on heating nickel in a stream of CO while the impurities remain unaffected. The compound on further heating decomposes to give pure nickel.

Thus, CO helps to purify nickel.
(ii) During roasting, the copper pyrites are converted into a mixture of Cu2O and FeO.
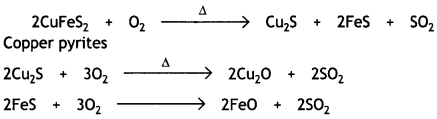
To remove FeO (basic), the roasted ore is mixed with silica and heated. Silica acts as a flux and combines with FeO to form fusible slag of iron silicate.
FeO + SiO2 → FeSiO3
The slag being lighter floats and forms the upper layer and is removed. Thus, silica helps to remove FeO.
(iii) Very reactive metals such as sodium, potassium, calcium, aluminium, etc. are extracted by electrolytic methods. Certain less reactive metals such as copper are also purified by using electro-refining method.
Question 16.
Write down the reactions taking place in blast furnace related to the metallurgy of iron in the temperature range 500 K to 800 K. What is the role of limestone in the metallurgy of iron?
OR
What happens when
(a) Silver is leached with NaCN in the presence of air?
(b) Copper matte is charged into silica-lined converter and hot air blast is blown?
(c) NaCN is added in an ore containing PbS and ZnS during concentration by froth floatation method? (CBSE Delhi Al 2019)
Answer:
Reactions taking place in blast furnace at 500K to 800K: The oxides of iron are reduced by CO.
FeO + CO → Fe + CO2
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + 4CO → 3Fe + 4CO2
Fe2O3 + CO → 2FeO + CO2
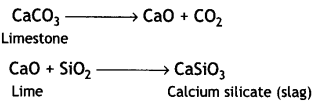
Role of Limestone: It acts as a flux which decomposes to calcium oxide.
CaCO3 → CaO + CO2
Limestone
CaO combines with impurity (e.g. Si02) to form slag which is then removed.
CaO + SiO2 → CaSiO3
OR
(a) Silver is leached with dilute solution (0.5%) of NaCN in the presence of atmospheric oxygen. The metal dissolves forming the complex.
4Ag + 8CN– + 2H2O + O2 → 4[Ag(CN)2]– + 4OH–
The metal is extracted from water-soluble complex by zinc metal.
2[Ag(CN)2]– + Zn → [Zn(CN)4]2- + 2Ag
(b) The copper matte containing Cu2S and FeS is put in silica-lined convertor and hot air blast is blown to convert remaining FeS to FeO, which is removed as slag with silica.
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO3
Cu2S or CuO gets converted to copper
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
(c) NaCN acts as a depressant in preventing ZnS from forming the froth. If forms a layer of zinc complex Na2[Zn(CN)4] on the surface of ZnS and thereby prevents it from forming the froth.
4NaCN + ZnS → Na2[Zn(CN)4] + Na2S
Long Answer Type Questions (LA-II)
Question 1.
Explain the following:
(i) Although thermodynamically feasible, in practice magnesium metal is not used for the reduction of alumina in the metallurgy of aluminium. Why?
(ii) Why is zinc and not copper used for the recovery of silver from the complex [Ag(CN)2]–?
(iii) The extraction of Au by leaching with NaCN involves both oxidation and reduction. Justify giving equations. (CBSE Sample Paper 2011)
(iv) Lime-stone is used in the manufacture of pig iron from haematite. Why?
Answer:
(i) Inspection of Ellingham diagram shows that ∆G vs T curves for Al2O3 and MgO intersect at a point corresponding to very high temperature of the order of 2000 K. This means above this temperature, ∆G for the reaction:
Al2O3 + 3Mg → 2Al + 3Mgo
would become negative and hence reduction will be feasible. However, this temperature is very high so that the process is uneconomical and technologically difficult.
(ii) Zinc is a stronger reducing agent (E° = – 0.76 V) and more electropositive than copper (E° = + 0.34 V). Therefore, zinc is used for recovery of Ag from [Ag(CN2)]– complex.
(iii) During leaching process, gold (Au) is first oxidised by O2 of the air to Au+ which then combines with CN– ions to form the soluble complex, dicyanoaurate (I).
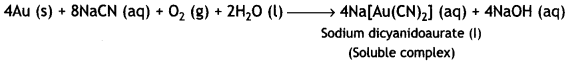
Gold is then extracted from this complex by displacement method by using a more electropositive zinc metal. In this method, zinc acts as a reducing agent and it reduces Au+ to Au. Zinc itself gets oxidised to Zn2+ ions which combine with CN– ions to form soluble complex, sodium tetracyanozincate (II).
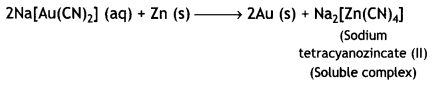
Thus, extraction of gold by leaching with NaCN involves both oxidation and reduction.
(iv) Haematite is an ore of iron and contains silica (SiO2) as the main impurity. The purpose of limestone is to remove SiO2 as calcium silicate (CaSiO3) slag.
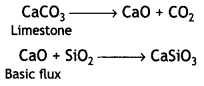
Question 2.
Name the principal ore of aluminium. Explain the significance of leaching in the extraction of aluminium. (CBSE AI 2013)
Answer:
The principal ore of aluminium is bauxite, Al2O3.2H2O. Leaching process is used to concentrate the ore of aluminium, bauxite, which is contaminated with impurities of silica (SiO2), iron oxide (Fe2O3), titanium oxide (TiO2), etc. Leaching is done by treating the powdered ore with hot cone. (45%) solution of NaOH at about 473-523 K and 35-36 bar pressure.
Al2O3.2H2O(s) + 2NaOH(aq) + H2O(l) → 2Na [Al(OH)4](aq)
The impurities of ferric oxide and silica are insoluble and are removed by filtration.
The solution containing sodium aluminate is neutralised by passing C02 gas and hydrated alumina is precipitated.
2Na [Al(OH)4] (aq) + CO2 (g) → Al2O3. x H2O (s) + 2Na HCO3(s)
Hydrated alumina is heated to 1473 K to get pure alumina.
![]()
Question 3.
Describe the principle controlling each of the following processes:
(i) Vapour phase refining of titanium metal.
(ii) Froth floatation method of concentration of sulphide ore.
(iii) Recovery of silver after silver ore was leached with NaCN. (CBSE AI 2011, CBSE Delhi 2011)
Answer:
(i) The principle of vapour phase refining is that certain metals are converted to their volatile compounds while the impurities are not affected during compound formation. The compound formed decomposes on heating to give pure metal. During refining of titanium metal, the titanium metal is heated with l2 to form a volatile compound Til4, which on further heating at higher temperature decomposes to give pure titanium metal.
![]()
(ii) This is based upon the different wetting properties of the ore and the gangue particles with oil and water. The mineral particles become wet by oil and the gangue particles by water. When the ore is mixed with water containing small quantities of pine oil and then by agitating the water by blowing air violently, the froth is formed. The froth carries the lighter ore particles to the surface and the heavier impurities sink to the bottom.
(iii) This is a chemical method and is useful for the ores which are soluble in certain suitable solvents but the impurities are not soluble. The impurities left undissolved are removed by filtration. The ore of silver, Ag2S (argentite), reacts with NaCN as
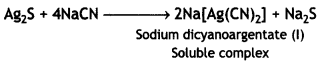
Sodium sulphide thus formed is oxidised to sodium sulphate by blowing air into the solution. This helps the reaction to occur in the forward direction.
4Na2S + 5O2 + 2H2O → 2Na2SO4 + 4NaOH + 2S
The above solution after filtration and removing insoluble impurities is heated with zinc to get silver.
![]()
Question 4.
Describe the role of the following:
(i) NaCN in the extraction of silver from a silver ore
(ii) Iodine in the refining of titanium
(iii) Cryolite in the metallurgy of aluminium (CBSE 2010)
Answer:
(i) The role of NaCN in the extraction of silver is to do the leaching of silver ore in the presence of atmospheric air from which silver is obtained later by replacement with zinc as:
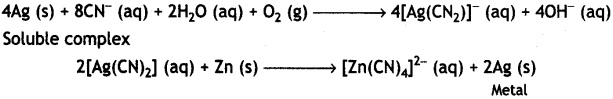
(ii) Iodine is used to form a volatile unstable compound of the metal, which is decomposed to get the pure metal. For example, titanium is heated with iodine to 523 K forming unstable titanium iodide which is decomposed to Ti as
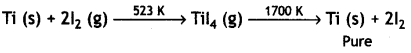
(iii) Cryolite is added to bauxite ore before electrolysis because of the following reasons:
(a) It acts as a solvent.
(b) It lowers the melting point of alumina to about 1173 K.
(c) Addition of cryolite to alumina increases the electrical conductivity.
Question 5.
Describe the role of the following:
(i) SiO2 in the extraction of copper from copper matte
(ii) NaCN in froth floatation process
Answer:
(i) The copper matte containing Cu2S and FeS is put in silica lined converter. Some silica is also added and hot air blast is blown to convert remaining FeS to FeO, which is removed as slag with silica.
2FeS + O2 → 2FeO + 2SO2
FeO + Si02 → FeSiO3
Cu2S or CuO gets converted to copper.
2Cu2S + 3O2 → 2Cu2O + 2SO2
2Cu2O + Cu2S → 6Cu + SO2
(ii) NaCN in froth floatation process is used to separate one sulphide ore from another and is known as depressant.
For example, sodium cyanide can be used as a depressant in the separation of zinc sulphide ore (ZnS) and lead sulphide ore (PbS). Sodium cyanide forms a layer of zinc complex, Na2[Zn(CN)4], on the surface of ZnS and therefore, prevents it from forming the froth. Therefore, it acts as a depressant.
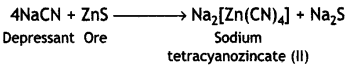
However, NaCN does not prevent PbS from forming the froth. Thus, it selectively prevents ZnS from coming to the froth but allows PbS to come with the froth. Thus, the two ores can be separated by the use of a depressant.