Here we are providing Class 12 Chemistry Important Extra Questions and Answers Chapter 8 The d-and f-Block Elements. Class 12 Chemistry Important Questions are the best resource for students which helps in Class 12 board exams.
Class 12 Chemistry Chapter 8 Important Extra Questions The d-and f-Block Elements
The d-and f-Block Elements Important Extra Questions Very Short Answer Type
Question 1.
How would you account for the increasing oxidising power in the series:
V02+ < Cr207 2-< Mn04–? (CBSE Sample Paper 2010)
Answer:
This is due to the increasing stability of the lower species to which they are reduced.
Question 2.
Which metal in the first transition series exhibits a +1 oxidation state most frequently and why? (CBSE Delhi 2013)
Answer:
Copper has electronic configuration 3cf104s1. It can easily lose one (4s1) electron to give stable 3d10 configuration.
Question 3.
The magnetic moments of a few transition metal ions are given below:
| Metal ion | Magnetic moment (BM) |
| Sc3+ | 0.00 |
| Cr2+ | 4.90 |
| Ni2+ | 2.84 |
| Ti3+ | 1.73 |
(atomic no. Sc = 21, Ti = 22, Cr = 24, Ni = 28)
Which of the given metal ions:
(i) has the maximum number of unpaired electrons?
Answer:
Cr2+
(ii) forms colourless aqueous solution?
Answer:
Sc3+
(iii) exhibits the most stable +3 oxidation state? (CBSE Sample Paper 2017-18)
Answer:
Sc3+
Question 4.
Based on the data, arrange Fe2+, Mn2+ and Cr2+ in the increasing order of stability of +2 oxidation state:
E°Cr3+/Cr2+ = – 0.4 V, E°Mn3+/Mn2+ = 1.5 V, E°Fe3+/Fe2+ = 0.8 V (CBSE Sample Paper 2011)
Answer:
As the value of reduction potential increases, the stability of +2 oxidation state increases. Therefore, correct order of stability is Cr3+ | Cr2+ < Fe3+ | Fe2+ < Mn3+ | Mn2+.
Question 5.
(i) Name the element showing the maximum number of oxidation states among the first series of transition metals from Sc (Z = 21) to Zn (Z = 30).
Answer:
Manganese
(ii) Name the element which shows only +3 oxidation state. (CBSEAI2013)
Answer:
Scandium
Question 6.
Identify the oxoanion of chromium which is stable in an acidic medium. (CBSE Sample Paper 2017-18)
Answer:
Cr2072-
Question 7.
Actinoid contraction is greater from element to element than lanthanoid contraction. Why? (CBSE Delhi 2015)
Answer:
This is because of relatively poor shielding by 5f electrons in actinoids in comparison with shielding of 4f electrons in lanthanoids.
Question 8.
Name an important alloy that contains some of the Ianthanoid metals. (CBSE AI 2013)
Answer:
Misch metal
Question 9.
Identify the Ianthanoid element that exhibits a +4 oxidation state. (CBSE Sample Paper 2017-18)
Answer:
Cerium
Question 10.
Write the formula of an oxo-anion of chromium (Cr) in which it shows the oxidation state equal to its group number. (CBSE Delhi 2017)
Answer:
Cr2072-
The d-and f-Block Elements Important Extra Questions Short Answer Type
Question 1.
Assign reasons for the following:
(i) Cu(I) Is not known In an aqueous solution.
Answer:
Because of the Lesser hydration enthalpy of Cu(I), It Is unstable In an aqueous solution and therefore, It undergoes disproportionation.
(ii) Actinolds exhibit a greater range of oxidation states than lanthanoids. (CBSE AI 2011)
Answer:
Lanthanoids show Limited number of oxidation states, such as + 2, + 3 and + 4 + 3 is the principal oxidation state). This is because of the large energy gap between 5d and 4f subshells. On the other hand, actinoids also show a principal oxidation state of + 3 but show a number of other oxidation states also. For example, uranium (Z = 92) exhibits oxidation states of + 3, + 4, + 5, + 6 and + 7 and neptunium (Z = 94) shows oxidation states of + 3, +4, + 5, + 6 and + 7. This is because of the small energy difference between 5f and 6d orbitals.
Question 2.
Give reasons:
(a) MnO is basic whereas Mn2O7 is acidic in nature.
Answer:
When a metal is in a high oxidation state, its oxide Is acidic and when a metaL is in a low oxidation state its oxide is basic.
The oxides of manganese have the following behavior:
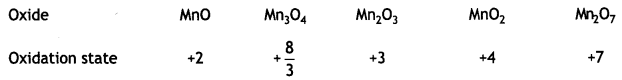
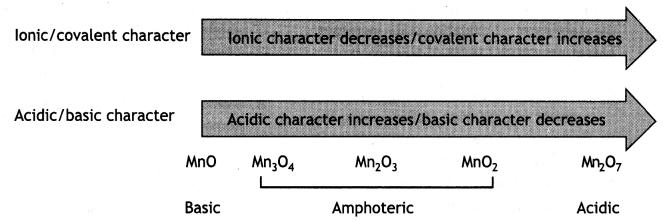
(b) Transition metals form alloys. (CBSE 2019C)
Answer:
The transition metals are quite similar in size and, therefore, the atoms of one metal can substitute the atoms of other metal in its crystal lattice. Thus, on cooling a mixture solution of two or more transition metals, solid alloys are formed which is shown in Fig.
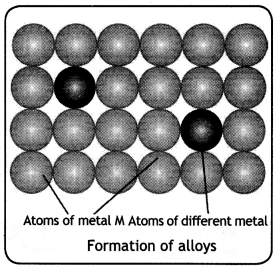
Question 3.
Explain why the Eθ value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+ or Fe3+/Fe2+. (CBSE AI 2008, 2010, 2018)
Answer:
Mn2+ has 3d5 electronic configuration. It is stable because of the half-filled configuration of the d-subshell. Therefore, Mn has a very high third ionization enthalpy for the change from d5 to d4 and it is responsible for a much more positive Eθ value for Mn3+/Mn2+ couple in comparison to Cr3+/Cr2+ and Fe3+/ Fe2+ couples.
Question 4.
Complete the following chemical equations:
(i) Cr2O72++ H+ + I– →
Answer:
Cr2O72+ + 6I– + 14H+ → 2Cr3+ + 3I2 + 7H2O
(ii) MnO4– + NO2– + H+ → (CBSE Delhi 2012)
Answer:
2MnO4– + 5N02– + 6H+ → 2Mn2+ + 5NO3–+ 3H2O
Question 5.
(i) Which metal in the first transition series (3d series) exhibits +1 oxidatIon state most frequently and why?
Answer:
Copper, because it has [Ar] 3d104s1 electronic configuration. After losing one electron it gets completely filled electronic configuration (3d10).
(ii) Which of the following cations are colored in aqueous solutions and why?
Sc3+, V3+, Ti4+, Mn2+
(At. nos. Sc = 21, V = 23, Ti = 22, Mn = 25) (CBSE Delhi 2013)
Answer:
Sc3+: [Ar] V3+: [Ar] 3d2
Ti4+: [Ar] Mn2+: [Ar] 3d5
V3+ and Mn2+ cations are colored because they contain partially filled d-orbitals.
Question 6.
Why do transition elements exhibit higher enthalpies of atomization? (CBSE Delhi 2008, 2012)
Answer:
The high enthalpies of atomization are due to a large number of unpaired electrons in their atoms. Therefore, they have stronger interatomic interactions and hence, stronger bonding between atoms. Thus, they have high enthalpies of atomization.
Question 7.
(a) Actinoid contraction is greater than lanthanoid contraction. Give reason,
Answer:
This is due to poorer shielding by 5f electrons in actinoids as compared to shielding by 4f electrons in lanthanoids. In the Ianthanoid series, as we move from one element to another, the nuclear charge increases by one unit, and one electron are added. The new electrons are added to the same inner 4f-subshells. However, the 4f-electrons shield each other from the nuclear charge quite poorly because of the very diffused shapes of the f-orbitals.
The nuclear charge, however, increases by one at each step. Hence, with increasing atomic number and nuclear charge, the effective nuclear charge experienced by each 4f-electron increases. As a result, the whole of the 4f-electron shell contracts at each successive element, though the decrease is very small.
The actinoid contraction is due to the imperfect shielding of one 5felectron by another in the same subshell. Therefore, as we move along the series, the nuclear charge and the number of 5f-electrons increase by one unit at each step. However, due to imperfect shielding of 5f orbitals, the effective nuclear charge increases which results in contraction of the size.
(b) Out of Fe and Cu, which has a higher melting point and why? (CBSE 2019C)
Answer:
Fe has a higher melting point. The metallic bond is formed due to the interaction of electrons in the outermost orbitals. The strength of bonding is related to the number of unpaired electrons. Fe has more unpaired electrons leading to stronger metallic bonding. So, it has a higher melting point.
Question 8.
How would you account for the following:
(i) Cr2+ is reducing in nature while with the same d-orbital configuration (d4), Mn3+ is an oxidizing agent.
Answer:
Cr2+ is reducing as its configuration changes from d4 to d3, the latter having a half-filled t2g level. On the other hand, the change from Mn3+ to Mn2+ results in the half-filled (d5) configuration which has extra stability.
(ii) In a transition series of metals, the metal which exhibits the greatest number of oxidation states occurs in the middle of the series. (CBSE 2011)
Answer:
This is because, in the middle of the transition series, the maximum number of electrons are available for sharing with others. The small number of oxidation states at the extreme left side is due to the lesser number of electrons to lose or share. On the other hand, at the extreme right-hand side, due to a large number of electrons, only a few orbitals are available in which the electrons can share with others for higher valence.
Question 9.
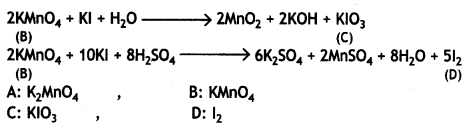
When MnO2 is fused with KOH in the presence of KNO3 as an oxidizing agent, it gives a dark green compound (A). Compound (A) disproportionates in an acidic solution to give a purple compound (B). An alkaline solution of compound (B) oxidizes Kl to compound (C), whereas an acidified solution of compound (B) oxidizes Kl to (D). Identify (A), (B), (C), and (D). (CBSE Delhi 2019)
Answer:
When MnO2 is fused with KOH in the presence of KNO3, as an oxidizing agent, it gives a dark green compound (A).
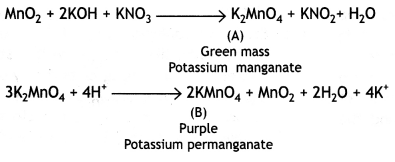
Question 10.
State reasons for the following:
(i) Cu(I) is not stable in an aqueous solution.
Answer:
Cu+ ion is not stable in an aqueous solution because of its less negative enthalpy of hydration than that of Cu2+ ion.
(ii) Unlike Cr3+, Mn2+, Fe3+ and the subsequent other M2+ ions of the 3d series of elements, the Ad and the 5d series metals generally do not form stable atomic species. (CBSE 2011)
Answer:
Because of lanthanoid contraction, the expected increase in size does not occur.
Question 11.
Assign reasons for each of the following:
(i) Transition metals generally form colored compounds.
Answer:
Most of the transition metal ions are colored both in the solid-state and in aqueous solutions. The color of these ions is attributed to the presence of an incomplete (n – 1) d-subshell. The electrons in these metal ions can be easily promoted from one energy level to another in the same d-subshell. The amount of energy required to excite the electrons to higher energy states within the same d-subshell corresponds to the energy of certain colors of visible light. Therefore, when white light falls on a transition metal compound, some of its energy corresponding to a certain color is absorbed causing the promotion of d-electrons. This is known as d-d transitions. The remaining colors of white light are transmitted and the compound appears colored.
(ii) Manganese exhibits the highest oxidation state of +7 among the 3d-series of transition elements. (CBSE Delhi 2011)
Answer:
Manganese has the electronic configuration [Ar] 3d5 4s2. It can lose seven electrons due to the participation of 3d and 4s electrons and therefore, exhibits the highest oxidation state of +7 in its compounds.
Question 12.
What are the transition elements? Write two characteristics of the transition elements. (CBSE Delhi 2015)
Answer:
Transition elements are those elements that have incompletely filled (partly filled) d-subshell in their ground state or in any one of their oxidation states.
Characteristics:
- Transition elements show variable oxidation states.
- They exhibit catalytic properties.
Question 13.
Though both Cr2+ and Mn3+ have d4 configurations, yet Cr2+ is reducing while Mn3+ is oxidizing. Explain why? (CBSE AI 2008, 2012, CBSE Delhi 2012)
Answer:
E° value for Cr3+/Cr2+ is negative (-0.41V), this means that Cr3+ ions are more stable than Cr2+. Therefore, Cr2+ can readily lose electrons to undergo oxidation to form Cr3+ ion, and hence Cr (II) is strongly reducing. On the other hand, the E° value for Mn3+ Mn2+ is positive (+1.57V), this means that Mn3+ ions can be readily reduced to Mn2+ and hence Mn (III) is strongly oxidizing.
Question 14.
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state? (CBSE Delhi 2012)
Answer:
The electronic configuration of Mn2+ is [Ar] 3d5 which is half-filled and hence it is stable. Therefore, the third ionization enthalpy is very high, i.e. the third electron1 cannot be easily removed. In the case of Fe2+, the electronic configuration is 3d6. Therefore, Fe2+ can easily lose one electron to acquire a 3d5 stable electronic configuration.
Question 15.
The E°M2+|M for copper is positive (0.34 V). Copper is the only metal in the first series of transition elements showing this behavior. Why? (CBSE Sample Paper 2012, CBSE AI2012)
Answer:
The E°M2+|M value for copper is positive and this shows that it is the least reactive metal among the elements of the first transition series. This is because copper has a high enthalpy of atomization and enthalpy of ionization. Therefore, the high energy required to convert Cu(s) to Cu2+ (aq) is not balanced by its hydration enthalpy.
Question 16.
Chromium is a typical hard metal while mercury is a liquid. (CBSE Sample Paper 2011)
Answer:
In chromium, the M-M interactions are strong due to the presence of six unpaired electrons in the 3d and 4s subshell. On the other hand, in mercury, all the electrons in the 5d and 6s subshell are paired and therefore, the M-M interactions are weak. Therefore, chromium is a typical hard metal while mercury is a liquid.
Question 17.
Silver is a transition metal but zinc is not. (CBSE Sample Paper 2011)
Answer:
According to the definition, transition elements are those which have partially filled d-subshell in their elementary state or in one of the oxidation states. Silver (Z = 47) can exhibit a +2 oxidation state in which it has an incompletely filled d-subshell (4d9 configuration). Hence, silver is regarded as a transition element.
On the other hand, zinc (Z = 30) has the configuration 3d10 4s2. It does not have partially filled d-subshells in its elementary form or in a commonly occurring oxidation state (Zn2+: 3d10). Therefore, it is not regarded as a transition element.
Question 18.
Name the ox metal anions of the first series of the transition metals in which the metal exhibits an oxidation state equal to its group number. (CBSE Delhi 2017)
Answer:
MnO4–: Oxidation state of Mn = +7 (equal to its group number)
CrO42-: Oxidation state of Cr = +6 (equal to its group number)
Question 19.
Give reasons:
(a) Of the d4 species, Cr2+ is strongly reducing while Mn3+ is strongly oxidising.
Answer:
Because Cr is more stable in the +3 oxidation state due to the t2g3 configuration whereas Mn is more stable in the +2 oxidation state due to the half-filled 3d5 configuration.
E° value for Cr3+/Cr2+ is negative (-0.41 V); this means that Cr3+ ions are more stable than Cr2+. Therefore, Cr2+ can readily lose electrons to undergo oxidation to form a Cr3+ ion, and hence Cr (II) is strongly reducing. On the other hand, the E° value for Mn3+/ Mn2+ is positive (+1.57V), which means that Mn3+ ions can be readily reduced to Mn2+ and hence Mn (III) is strongly oxidizing.
(b) The d1 configuration is very unstable in ions. (CBSE 2019C)
Answer:
After the loss of ns electrons, d1 electron can easily be lost to give a stable configuration. Therefore, the elements having d1 configuration are either reducing or undergo disproportionation.
Question 20.
When chromite ore FeCr2O4 is fused, with NaOH in presence of air, a yellow-colored compound (A) is obtained, which on acidification with dilute sulphuric acid gives a compound (B). Compound (B) on reaction with KCI forms an orange colored crystalline compound (C).
(i) Write the formulae of the compounds (A), (B), and (C).
(ii) Write one use of the compound (C). (CBSE Delhi 2016)
OR
Complete the following chemical equations:
(i) 8MnO4–+ 3S2O32-+ H2O →
(ii) Cr2O72-+ 3Sn2+ + 14H+ →
Answer:
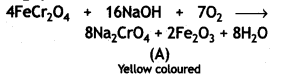
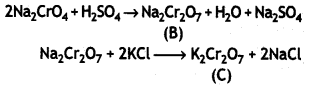
(ii) Potassium dichromate (C) is used as a powerful oxidizing agent in redox titrations in the laboratories.
OR
(i) 8MnO4–+ 3S2O32-+ H2O → 8MnO2 + 6SO42- + 2OH–
(ii) Cr2O7 2+ + 3Sn2+ +14H+ → 2Cr3+ + 3Sn4+ + 7H2O
Question 21.
Why do the transition elements have higher enthalpies of atomization? In 3d series (Sc to Zn), which element has the lowest enthalpy of atomization and why? (CBSE 2015)
Answer:
The transition elements have high enthalpies of atomization because they have a large number of unpaired electrons in their atoms. Therefore, they have stronger interatomic interactions and hence, stronger bonding between atoms. Thus, they have high enthalpies of atomization.
Zinc has the lowest enthalpy of atomization because it has no unpaired electrons and hence weak metallic bonding.
The d-and f-Block Elements Important Extra Questions Long Answer Type
Question 1.
(i) Transition metals and their compounds are generally found to be good catalysts.
(ii) Metal-metal bonding is more frequent for the 4d and the 5d series of transition metals than that for the 3d series. (CBSE AI 2011)
Answer:
(i) Some transition metals and their compounds act as good catalysts for various reactions. This is due to their ability to show multiple oxidation states. The common examples are Fe, Co, Ni, V, Cr, Mn, Pt, etc.
The transition metals form reaction intermediates with the substrate by using empty d-orbitals. These intermediates give reaction paths of lower activation energy and therefore, increase the rate of reaction. For example, during the conversion of SO2 to SO3, V2O5 is used as a catalyst. Solid V2O; absorbs a molecule of SO2 on the surface forming V2O4 and the oxygen is given to SO2 to form S03. The divanadium tetraoxide is then converted to V2O5 by reaction with oxygen:
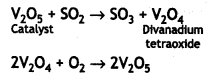
(ii) The second and third transition series metals show a great tendency to form strong M-M bonds than the first transition series elements. For example, rhenium easily forms ReCl84- which contains a strong Re-Re bond. It does not have a manganese analog.
Similarly, both Niobium and tantalum form M6X12n+ species and has no vanadium analogue.
Question 2.
Explain the following observations giving an appropriate reason for each.
(i) The enthalpies of atomization of transition elements are quite high.
Answer:
The high enthalpies of atomization are due to a large number of unpaired electrons in their atoms. Therefore, they have stronger interatomic interaction and hence, stronger bonding between atoms. Thus, they have high enthalpies of atomization.
(ii) There occurs much more frequent metal-metal bonding in compounds of heavy transition metals (i.e. 3rd series)
Answer:
The heavier transition metals of second and third transition series metals show a great tendency to form strong M-M bonds than first transition series elements. For example, rhenium easily forms ReCl84- which contains a strong Re-Re bond. It does not have a manganese analog. Similarly, both niobium and tantalum form M6X12n+ species and has no vanadium analog.
(iii) Mn2+ is much more resistant than Fe2+ towards oxidation. (CBSE Delhi 2012)
Answer:
Mn2+ is quite stable because it has a stable half-filled d5 electronic configuration and therefore, cannot be easily oxidized. On the other hand, Fe2+ has a less stable d6 electronic configuration and can be easily oxidized to a stable d5 configuration.
Question 3.
How would you account for the following?
(i) Transition metals exhibit variable oxidation states.
(ii) Zr (Z = 40) and Hf (Z = 72) have almost identical radii.
(iii) Transition metals and their compounds act as a catalyst.
OR
Complete the following chemical equations:
(i) Cr2072- + 6Fe2+ + 14H+ →
(ii) 2Cr042- + 2H+ →
(iii) 2Mn04– + 5C2042- + 16H+ → (CBSE Delhi 2013)
Answer:
(i) The transition elements exhibit variable oxidation states. The variable oxidation states of transition metals are due to the participation of ns and (n – 1) d-electrons. This is because of the very small difference between the energies of (n -1) d and ns orbitals. For the first five elements, the minimum oxidation state is equal to the number of electrons in the 4s orbitals and the other oxidation states are equal to the sum of 4s and some of the 3d-electrons. The highest oxidation state is equal to the sum of 4s and 3d electrons. For the remaining elements, the minimum oxidation state is equal to electrons in 4s-orbitals and the maximum oxidation state is not equal to the sum of 4s and 3d electrons.
In general, the oxidation state increases up to the middle and then decreases.
(ii) Due to lanthanoid contraction, the increase in radii from the second to third transition series vanishes. Therefore, Zr and Hf have almost the same radii.
(iii) Catalytic properties of transition metal ions. Some transition metals and their compounds act as good catalysts for various reactions. The common examples are Fe, Co, Ni, V, Cr, Mn, Pt, etc. For example, iron-molybdenum is used as a catalyst in Haber’s process for the manufacture of NH3. V205 is used for the oxidation of S02 to S03 in the Contact process for the manufacture of H2S04.
The transition metals form reaction intermediates with the substrate by using empty d-orbitals. These intermediates give reaction paths of lower activation energy and therefore, increase the rate of reaction. For example, during the conversion of SO2 to SO3, V2O5 is used as a catalyst. Solid V2O5 absorbs a molecule of SO2 on the surface forming V2O4 and the oxygen is given to SO2 to form SO3. The divanadium tetraoxide is then converted to V2O5 by reaction with oxygen:
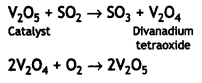
OR
(i) 6Fe2+ + Cr2072- + 14H+ → 6Fe3+ + 2Cr3+ + 7H2O
(ii) 2CrO42- + 2H+ → Cr2O72-+ H2O
(iii) 2MnO4 –+ 5C2O42– + 16H+ → 2Mn2+ + 8H2O + 10CO2
Question 4.
Give reasons for the following:
(i) Transition elements and their compounds act as catalysts.
Answer:
Transition metals and their compounds act as good catalysts because of their ability to show multiple oxidation states and form complexes, for example, Fe, Co, Ni, V, Cr, V2O5, etc.
(ii) E° value for (Mn2+|Mn) is negative whereas for (Cu2+| Cu) is positive.
Answer:
The negative value of E° (Mn2+| Mn) is due to the stability of the half-filled d-subshell in Mn2+(3d5). The positive E°(Cu2+|Cu) is due to the high ionization enthalpy and high enthalpy of atomization of copper. Therefore, the high energy required to convert Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(iii) Actinoids show irregularities in their electronic configuration. (CBSE Delhi 2019)
Answer:
Actinoids have irregularities in the electronic configuration because of almost equal energy of 5f, 6d and 7s orbitals. Therefore, there are some irregularities in the filling of 5f, 6d, and 7s orbitals. The electron may enter either of these orbitals.
Question 5.
(a) Complete the following chemical reactions:
(i) Na2Cr2O7 + KCl →
(ii) 2MnO4–+ 5 S032- + 6 H+ →
Answer:
(i) Na2Cr2O7 + KCl → K2Cr2O7 + 2 NaCl
(ii) 5 S032- + 2MnO4– + 6 H+ → 2Mn2+ + 3H2O + 5SO42-
(b) How does the colour of Cr2O72- change when treated with an alkali? (CBSE2019C)
Answer:
The orange color of Cr2O72- change to yellow due to the formation of chromate ion.
Cr2O72- + 2OH– → 2Cr2O72- + H2O (Yellow)
Question 6.
(a) Write chemical equations involved in the preparation of KMn04 from Mn02.
Answer:
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO42-+ 4H+ → 2MnO–+ MnO2 + 2H2O (or any other correct equation of preparation)
(b) Actinoids show wide range of oxidation states. Why? (CBSE 2019C)
Answer:
Lanthanoids show limited number of oxidation states, such as + 2, + 3 and + 4 (+ 3 is the principal oxidation state). This is because of large energy gap between 5d and 4f subshells. On the other hand, actinoids also show principal oxidation state of + 3 but show a number of other oxidation states also. For example, uranium (Z = 92) exhibits oxidation states of + 3, + 4, + 5 and + 6 and neptunium (Z = 93) shows oxidation states of + 3, + 4, + 5, + 6 and + 7.
This is because of the small energy difference between 5f, 6d, and 7s orbitals.
Question 7.
How would you account for the following?
(i) Many of the transition elements are known to form interstitial compounds.
Answer:
Transition metals form interstitial compounds. Transition metals have a unique character to form interstitial compounds with small non-metallic elements such as hydrogen, boron, carbon and nitrogen. The small atoms of these non-metallic elements (H, B, C, N, etc.) fit into the vacant spaces of the lattices of the transition metal atoms. As a result of the filling up of the interstitial spaces, the transition metals become rigid and hard.
These interstitial compounds have similar chemical properties as the parent metals but have different physical properties, particularly, density, hardness, and conductivity. For example, steel and cast iron are hard because of the formation of interstitial compounds with carbon. These interstitial compounds have a variable composition and cannot be expressed by a simple formula. Therefore, these are called nonstoichiometric compounds.
(ii) The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group members of the second (4d) series.
Answer:
The metallic radii of the third (5d) series of transition metals are virtually the same as those of corresponding group members of the (4d) series due to the phenomenon of lanthanoid contraction. The steady decrease in atomic and ionic sizes of lanthanoid elements with increasing atomic numbers is known as lanthanoid contraction.
(iii) Lanthanoids form primarily +3 ions, white the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical. (CBSE Delhi 2012)
Answer:
Lanthanoids form primarily +3 ions, while actinoids usually have higher oxidation states, +4 or even +6 in their compounds because in actinoids the 5f, 6d, and 7s energy levels are of comparable energies.
Therefore, all these three subshells can participate.
Question 8.
How would you account for the following:
(i) Among lanthanoids, Ln (III) compounds are predominant. However, occasionally in solutions or in solid compounds, +2 and +4 ions are also obtained.
Answer:
All lanthanoids exhibit a common stable oxidation state of +3. In addition, some lanthanoids show +2 and +4 oxidation states also in solution or in solid compounds. These are shown by these elements which by doing so attain the stable f (empty f-subshell), f (half-filled f-subshell), and f4 (filled f-subshell) configurations. For example,
(a) Ce and Tb exhibit +4 oxidation states. Cerium (Ce) and terbium (Tb) attain f and f configurations respectively when they get +4 oxidation state, as shown below:
Ce4+: [Xe] 4f°
Tb4+: [Xe] 4 f°
(b) Eu and Yb exhibit + 2 oxidation states.
Europium and ytterbium get f and f4 configurations in +2 oxidation state as shown below:
EU2+ : [Xe] 4 f7
Yb2+: [Xe] 4f14
(ii) The E°M2+/M for copper is positive (0.34 V). Copper is the only metal in the first series of transition elements showing this behavior.
Answer:
The E°(M2+/M) value for copper is positive and this shows that it is the least reactive metal out of the first transition series. This is because copper has a high enthalpy of atomization and enthalpy of ionization. Therefore, the high energy required to convert Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(iii) The metallic radii of the third (5d) series of transition metals are nearly the same as those of the corresponding members of the second series. (CBSE 2012)
Answer:
The metallic radii of the third transition series elements are virtually the same as those of corresponding members of the second transition series because of the lanthanoid contraction. Due to the presence of lanthanoids between the second and third transition series, the expected increase in radii vanishes. Consequently, the pairs of elements Zr-Hf, Nb-Ta, Mo-W, etc have almost similar sizes.
Question 9.
Explain the following observations:
(i) Many of the transition elements are known to form interstitial compounds.
Answer:
Transition metals form interstitial compounds. Transition metals have a unique character to form interstitial compounds with small non-metallic elements such as hydrogen, boron, carbon, and nitrogen. The small atoms of these non-metallic elements (H, B, C, N, etc.) fit into the vacant spaces of the lattices of the transition metal atoms. As a result of the filling up of the interstitial spaces, the transition metals become rigid and hard.
These interstitial compounds have similar chemical properties as the parent metals but have different physical properties, particularly, density, hardness, and conductivity. For example, steel and cast iron are hard because of the formation of interstitial compounds with carbon. These interstitial compounds have a variable composition and cannot be expressed by a simple formula. Therefore, these are called nonstoichiometric compounds.
(ii) There is a general increase in density from titanium (Z = 22) to copper (Z = 29).
Answer:
There is a gradual increase in density from Ti to Cu because as we move along a transition series from left to right, the atomic radii decrease due to an increase in effective nuclear charge. Therefore, the atomic volume decreases but at the same time atomic mass increases. Therefore, density (mass/volume) increases.
(iii) The members of the actinoid series exhibit a larger number of oxidation states than the corresponding members of the lanthanoid series. (CBSE 2012)
Answer:
Lanthanoids show limited a number of oxidation states, such as + 2, + 3, and + 4 (+ 3 is the principal oxidation state). This is because of the large energy gap between 5d and 4/ subshells. On the other hand, actinoids also show a principal oxidation state of + 3 but show a number of other oxidation states also. For example, uranium (Z = 92) exhibits oxidation states of + 3, + 4, + 5 and + 6 and neptunium (Z = 93) shows oxidation states of + 3, + 4, + 5, + 6 and + 7. This is because of the small energy difference between 5f, 6d, and 7s orbitals.
Question 10.
Following are the transition metal ions of 3d series.
Ti4+, V2+, Mn3+, Cr3+
(Atomic numbers: Ti = 22, V = 23, Mn = 25, Cr = 24)
Answer the following:
(i) Which ion is most stable in an aqueous solution and why?
(ii) Which ion is a strong oxidizing agent and why?
(iii) Which ion is colorless and why? (CBSE AI 2017)
Answer:
(i) Cr3+ because of the half-filled t2g3 configuration.
(ii) Mn3+ due to stable d5 configuration of Mn2+
(iii) Ti4+ because it has no unpaired electrons.
Question 11.
Write chemical equations for the following reactions:
(i) Oxidation of nitrite ion by MnO4– in acidic medium.
(ii) Acidification of potassium chromate solution.
(iii) Disproportionation of manganese
(vi) in acidic solution. (CBSE Sample Paper 2011)
Answer:
(i) 5NO2– + 2MnO4– + 6H+ → 2Mn2+ + 3H2O + 5NO3–
(ii) 2K2CrO44 + 2H+ → K2Cr2O7 + 2K+ + H2O
(iii) 3MnO42- + 4H+ → 2MnO4– + MnO2 + 2H2O
Question 12.
The actinoids exhibit a larger number of oxidation states than the corresponding members in the lanthanoid series. (CBSE AI 2012, CBSE Delhi 2012)
Answer:
Lanthanoids show a limited number of oxidation states, such as +2, +3, and +4 (+3 is the principal oxidation state). This is because of the large energy gap between 5d and 4f subshells. On the other hand, actinoids also show a principal oxidation state of +3 but show a number of other oxidation states also. For example, uranium (Z = 92) exhibits oxidation states of +3, +4, +5 and +6 and neptunium (Z = 93) and plutonium (Z = 94) show oxidation states of +3, +4, +5, +6 and +7. This is because of the small energy difference between 5f, 6d, and 7s orbitals.
Question 13.
Explain the following:
(i) Out of Sc3+, Co2+, and Cr3+ ions, only Sc3+ is colorless in aqueous solutions.
(Atomic no.: Co = 27; Sc = 21 and Cr = 24)
(iI) The E°Cu2+/Cu for copper metal is positive (+0.34), unlike the remaining members of the first transition series
(iiI) La(OH)3 is more basic than Lu(OH)3. (CBSE Sample paper 2018)
Answer:
(i) Co2+: [Ar]3d7, Sc3+: [Ar]3d°
Cr3+: [Ar]3d3
Co2+ and Cr3+ have unpaired electrons. Thus, they are colored in an aqueous solution. Sc3+ has no unpaired electron. Thus it is colorless.
(ii) Metal copper has a high enthalpy of atomization and enthalpy of ionization. Therefore the high energy required to convert Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(iii) Due to lanthanoid contraction the size of lanthanoid ion decreases regularly with the increase in atomic size. Thus covalent character between lanthanoid ion and OH’ increases from La3+ to Lu3+. Thus the basic character of hydroxides decreases from La(OH)3 to Lu(OH)3
Question 14.
(a) Complete the following chemical reactions:
(i) 2MnO4–+ 5 NO2–+ 6 H+ →
(ii) 3Mn042- + 4 H+ →
Answer:
(i) 5NO2– + 2MnO4– + 6H+ → 2Mn2+ + 5NO3– + 3H2O
(ii) 3MnO42-+ 4H+ → 2MnO4– + MnO2 + 2H2O
(b) Name a member of the lanthanoid series which shows a +4 oxidation state. (CBSE 2019C)
Answer:
Cerium / Ce
Question 15.
Give reasons:
(i) E° value for Mn3+/Mn2+ couple is much more positive than that for Fe3+/Fe2+.
Answer:
The comparatively high value for Mn shows that Mn2+(d5) is particularly stable/Much larger third ionization energy of Mn (where the required change is from d5 to d4).
(ii) Iron has a higher enthalpy of atomization than copper.
Answer:
Due to the higher number of unpaired electrons.
(iii) Sc3+ is colorless in an aqueous solution whereas Ti3+ is colored. (CBSE 2018)
Answer:
The absence of unpaired d-electron in Sc3+ whereas in Ti3+ there is one unpaired electron or Ti3+ shows the d-d transition.
Question 16.
(i) Complete the following chemical equations for reactions:
(a) MnO4– (aq) + S2O32- (aq) + H2O (I) →
(b) Cr2O7– (aq) + H2S(g) + H+(aq) →
Answer:
(a) 8MnO4– (aq) + 3S2O32- (aq) + H2O (l) → 8MnO2 (s) + 6SO42- (aq) + 2OH- (aq)
(b) Cr2O72- (aq) + 3H2S(g) + 8H+ (aq) → 2Cr3+(aq) + 3S(s) + 7H2O
(ii) Give an explanation for each of the following observations:
(a) The gradual decrease in size (actinoid contraction) from element to element is greater among the actinoids than that among the lanthanoids (lanthanoid contraction).
Answer:
(a) This is because of relatively poor shielding by 5f electrons in actinoids in comparison with shielding of 4f electrons in lanthanoids.
(b) The greatest number of oxidation states are exhibited by the members in the middle of a transition series.
Answer:
This is because, in the middle of the transition series, the maximum number of electrons are available for sharing with others. The small number of oxidation states at the extreme left side is due to a lesser number of electrons to lose or share. On the other hand, at the extreme right-hand side, due to a large number of electrons, only a few orbitals are available in which the electrons can share with others for higher valence.
(c) With the same d-orbital configuration (d4) Cr2+ ion is a reducing agent but the Mn3+ ion is an oxidizing agent. (CBSE Delhi 2009)
Answer:
Cr2+ is reducing as its configuration changes from d4 to d3, the latter having a half-filled t2g level. On the other hand, the change from Mn3+ to Mn2+ results in the half-filled (d5) configuration which has extra stability.
Question 17.
(i) Complete the following chemical reaction equations:
(a) Fe2+ (aq) + MnO–4 (aq) + H+ (aq) →
(b) Cr2O72- (aq) + I– (aq) + H+ (aq) →
Answer:
(a) 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) → Mn2+ (aq) + 5Fe3+ (aq) + 4H2O(l)
(b) Cr2O72- (aq) + 6I– (aq) + 14H+ (aq) → 2Cr3+(aq) + 3I2 (s) + 7H2O(l)
(ii) Explain the following observations:
(a) Transition elements are known to form many interstitial compounds.
Answer:
Transition metals form interstitial compounds. Transition metals have a unique character to form interstitial compounds with small non-metallic elements such as hydrogen, boron, carbon, and nitrogen. The small atoms of these non-metallic elements (H, B, C, N, etc.) fit into the vacant spaces of the lattices of the transition metal atoms. As a result of the filling up of the interstitial spaces, the transition metals become rigid and hard.
These interstitial compounds have similar chemical properties as the parent metals but have different physical properties, particularly, density, hardness, and conductivity. For example, steel and cast iron are hard because of the formation of interstitial compounds with carbon. These interstitial compounds have a variable composition and cannot be expressed by a simple formula. Therefore, these are called nonstoichiometric compounds.
(b) With the same d4 d-orbital configuration Cr2+ ion is reducing while the Mn3+ ion is oxidizing.
Answer:
Cr2+ is reducing as its configuration changes from d4 to d3, the latter having a half-filled t2g level. On the other hand, the change from Mn3+ to Mn2+ results in the half-filled (d5) configuration which has extra stability.
(c) The enthalpies of atomization of the transition elements are quite high. (CBSE Delhi 2009)
Answer:
The transition metals have strong interactions of electrons in the outermost orbitals and therefore, have high melting and boiling points. These suggest that the atoms of these elements are held together by strong forces and have high enthalpy of atomization.
Question 18.
(i) Complete the following chemical equations:
(a) MnO4– (aq) + S2O32- (aq) + H2O(l) →
(b) Cr2O72- (aq) + Fe2+ (aq) + H+ (aq) →
Answer:
(a) 8MnO4– + 3S2O32- + H2O → 8MnO2 + 6SO42-+ 2OH-
(b) Cr2O72- + 14H+ + 6Fe2+ → 2Cr3+ +6Fe3+ + 7H2O
(ii) Explain the following observations:
(a) La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in solutions.
Answer:
In La3+ there are no f-electrons and in Lu3+ the 4f sub-shell is complete (4/14). Therefore, there are no unpaired electrons and consequently, d-d transitions are not possible. Hence, La3+ and LU3+ do not show any color in solutions.
(b) Among the divalent cations in the first series of transition elements, manganese exhibits the maximum paramagnetism.
Answer:
Among the divalent cations in the first
transition series, Mn (Z = 25) exhibits the maximum paramagnetism because it has the maximum number of unpaired electrons (3d5): five unpaired electrons.
(c) Cu+ ion is not known in aqueous solutions. (CBSE 2010)
Answer:
In an aqueous solution, Cu+ undergoes disproportionation changing to Cu2+ ion.
2Cu+ → Cu2+ + Cu
Question 19.
(i) Give reasons for the following:
(a) Mn3+ is a good oxidizing agent.
(b) E°M2+/M values are not regular for first-row transition metals (3d series).
(c) Although ‘F’ is more electronegative than ‘O’, the highest Mn fluoride is MnF4, whereas the highest oxide is Mn207.
(ii) Complete the following equations:
(a) 2CrO42- + 2H+ →
(b) KMnO4 ![]()
OR
(i) (a) Why do transition elements show variable oxidation states?
(b) Name the element showing a maximum number of oxidation states among the first series of transition metals from Sc(Z = 21) to Zn (Z = 30).
(c) Name the element which shows only the +3 oxidation state.
(ii) What is lanthanoid contraction? Name an important alloy that contains some of the lanthanoid metals. (CBSE2013)
Answer:
(i) (a) Mn3+ (3d4) on changing to Mn2+ (3d5) becomes stable, half-filled configuration has extra stability. Therefore, Mn3+ can be easily reduced and acts as a good oxidizing agent.
(b) E°(M2+/M) values are not regular in the first transition series metals because of irregular variation of ionization enthalpies (IE1 + IE2) and the sublimation energies.
(c) Among transition elements, the bonds formed in +2 and +3 oxidation states are mostly ionic. The compounds formed in higher oxidation states are generally formed by sharing of d-electrons. Therefore, Mn can form MnO4- which has multiple bonds also, while fluorine cannot form multiple bonds.
(ii) (a) 2CrO42- + 2H+ → Cr2O72- + H2O
(b) 2KMnO4 ![]() K2MnO4 + MnO2 + O2
K2MnO4 + MnO2 + O2
OR
(i) (a) The transition elements show variable oxidation states because their atoms can lose a different number of electrons. This is due to the participation of inner (n -1). d-electrons in addition to outer ns electrons because the energies of the ns and (n – 1) d-subshells are almost equal.
(b) Manganese
(c) Scandium
(ii) The steady decrease in atomic and ionic sizes of lanthanide elements with increasing atomic numbers is called lanthanide contraction. In the lanthanoids, there is a regular decrease in the size of atoms and ions with an increase in atomic number. For example, the ionic radii decrease from Ce3+ (111 pm) to Lu3+ (93 pm).
Cause of lanthanoid contraction. As we move through the lanthanoid series, 4f-electrons are being added, one at each step. The mutual shielding effect of electrons is very little, even smaller than that of d-electrons. This is due to the shape of f-orbitals. The nuclear charge, however, increases by one at each step. Hence, the inward pull experienced by the 4f-electrons increases. This causes a reduction in the size of the entire 4f shell. The sum of the successive reductions gives the total lanthanoid contraction. The important alloy is mischmetal.
Question 20.
(i) How do you prepare:
(a) K2MnO4 from MnO2?
(b) Na2Cr2O7 from Na2CrO4?
(ii) Account for the following:
(a) Mn2+ is more stable than Fe2+ towards oxidation to +3 state.
(b) The enthalpy of atomization is lowest for Zn in the 3d series of the transition elements.
(c) Actinoid elements show a wide range of oxidation states.
OR
(a) Name the element of 3d transition series which shows a maximum number of oxidation states. Why does it show so?
(b) Which transition metal of 3d series has a positive E°(M2+/M) value and why?
(c) Out of Cr3+ and Mn3+, which is a stronger oxidizing agent and why?
(d) Name a member of the lanthanoid series which is well known to exhibit a +2 oxidation state.
(e) Complete the following equation:
Mn04– + 8H+ +5e– → (CBSE Delhi 2014)
Answer:
(i) (a) When Mn02 is fused with caustic potash (KOH) or potassium carbonate in the presence of air, a green mass of potassium manganate is formed.
2MnO2 + 4KOH + O2 ![]() 4 2K2MnO4 + 2H2O
4 2K2MnO4 + 2H2O
Or
2MnO2 + 2K2CO2 + O2 → 2K2MnO4 + 2CO2.
(b) Na2Cr04 is acidified with dilute sulphuric acid to get Na2Cr2O7.
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
(ii) (a) Electronic configuration of Mn2+ is [Ar]Bd5 which is half-filled and hence is stable. Therefore, the third ionization enthalpy is very high, i.e. the third electron cannot be easily removed. In the case of Fe2+, the electronic configuration is 3d6. Therefore, Fe2+ can easily lose one electron to acquire a 3d5 stable configuration. Thus, Mn2+ is more stable than Fe2+ towards oxidation to + 3 states.
(b) The high enthalpies of atomization of transition elements are due to the participation of electrons (n – 1) d-orbitals in addition to ns electrons in the interatomic metallic bonding. In the case of zinc, no electrons from 3d-orbitals are involved in the formation of metallic bonds. On the other hand, in all other metals of 3d series electrons from d-orbitals are always involved in the formation of metallic bonds.
(c) Lanthanoids show a limited number of oxidation states, such as +2, +3, and +4 (+3 is the principal oxidation state). This is because of the large energy gap between 5d and 4/ subshells. On the other hand, actinoids also show a principal oxidation state of +3 but show a number of other oxidation states also. For example, uranium (Z = 92) exhibits oxidation states of +3, +4, +5 and +6 and neptunium (Z = 94) shows oxidation states of +3, +4, +5, +6 and +7. This is because of the small energy difference between 5f and 6d orbitals.
OR
(a) Manganese (Z = 25) shows the largest number of oxidation states because it has the maximum number of unpaired electrons. It shows oxidation states from +2 to +7, i.e. +2, +3, +4, +5, +6 and +7.
(b) The E°(M2+|M) value for copper is positive and this shows that it is the least reactive metal among the elements of the first transition series. This is because copper has a high enthalpy of atomization and enthalpy of ionization. Therefore, the high energy required to convert Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(c) Mn3+ is a stronger oxidizing agent than Cr3+ because Mn3+(3d4) changes to stable half-filled (3d5) configuration while in the case of Cr3+ (3d5) is stable (half-filled) and it cannot be readily changed to Cr2+ (3d4).
(d) Europium.
(e) MnO4– + 8H+ + 5e– → Mn2+ + 4H2O.
Question 21.
(a) Account for the following:
(i) Manganese shows the maximum number of oxidation states in 3d series.
(ii) E° value for Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+.
(iii) Ti4+ is colorless whereas V4+ is colored in an aqueous solution.
(b) Write the chemical equations for the preparation of KMn04 from Mn02. Why does the purple color of acidified permanganate solution decolorize when it oxidizes Fe2+ to Fe3+?
OR
(a) Write one difference between transition elements and p-block elements with reference to variability of oxidation states.
(b) Why do transition metals exhibit higher enthalpies of atomization?
(c) Name an element of the lanthanoid series which is well known to show a +4 oxidation state. Is it a strong oxidizing agent or a reducing agent?
(d) What is lanthanoid contraction? Write its one consequence.
(e) Write the ionic equation showing the oxidation of Fe(ll) salt by acidified dichromate solution. (CBSE Al 2019)
Answer:
(a) (i) Manganese has the electronic configuration: 3d5 4s2. It has a maximum number of unpaired electrons and hence shows maximum oxidation states.
(ii) Mn2+ has 3d5 electronic configuration. It is stable because of the half-filled configuration. Therefore, Mn3 easily gets reduced to Mn2+. Thus, E°(M3+/Mn2+) is positive. On the other hand, Cr3+ is more stable in the +3 oxidation state due to stable t2g3 configuration.
(iii) Ti4+ (3d°) does not have any d-electrons. Therefore, there are no d-d transitions whereas V4+, (3d1) has one electron in d-subshell and d-d transitions are possible and hence it is colored.
(b) 2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3K2MnO4 + 4HCL → 2KMnO + MnO2 + 2H2O
When acidified KMnO4 oxidizes Fe2+ to Fe3+ its purple color gets decolorized due to the formation of Mn2+ from
MnO4– ion.
MnO4– + 5Fe2+ + 8W → Mn2+ + 5Fe3+ + 4H2O
Or
(a) Transition elements, in general, show variable oxidation states which differ by 1 unit, whereas p-block elements show variable oxidation states which differ by 2 units.
(b) Transition eLements have unpaired d-electron and therefore have strong metallic bonding between atoms. Hence, they have high enthalpies of atomization.
(c) Cerium. It is a strong oxidizing agent.
(d) The regular decrease In atomic and ionic radii of the Lanthanides with increasing atomic number is catted Lanthanoid contraction.
Consequence: 5d series elements have almost the same size as the 4d series.
(e) 6Fe2+ + Cr2O72- + 14H+ → 6Fe3+ + 2Cr3+ + 7H2O
Question 22.
(i) Account for the following:
(a) Mn shows the highest oxidation state of +7 with oxygen but with fluorine, it shows the highest oxidation state of +4.
(b) Zirconium and Hafnium exhibit similar properties.
(c) Transition metals act as catalysts.
(ii) Complete the following equations:
(a) 2MnO2 + 4KOH + O2 ![]()
(b) Cr2O72- + 14H+ + 6I–
Or
The elements of 3d transition series are given as:
Sc Ti V Cr Mn Fe Co Ni Cu Zn
Answer the following:
(a) Write the element which is not regarded as a transition element. Give reason.
(b) Which element has the highest m.p.?
(c) Write the element which can show an oxidation state of +1.
(d) Which element is a strong oxidizing agent in the +3 oxidation state and why? (CBSE 2016)
Answer:
(i) (a) Manganese shows the highest oxidation state of +7 with oxygen but +4 with fluorine. This is because oxygen has a tendency to form multiple bonds and hence stabilize the high oxidation state.
(b) Due to lanthanoid contraction, Zr and Hf show similar properties.
(c) The transition metals act as catalysts. This is due to their ability to show multiple oxidation states.
(ii) (a) 2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
(b) Cr2O72- + 14H+ + 6I– → 2Cr3+ + 7H2O + 3I2
Or
(a) Zn because of not having partially filled d-orbitals in its ground state or ionic state.
(b) Chromium, Cr
(c) Copper, Cu
(d) Mn, because Mn2+ has extra stability due to half-filled d-subshell.
Mn: [Ar] 3d5 4s2; Mn2+: [Ar]3d5
Mn3+ has four electrons (3d4) in 3d subshell and requires only one electron to get a half-filled 3d subshell and therefore, acts as a strong oxidizing agent.