By going through these CBSE Class 12 Chemistry Notes Chapter 10 Haloalkanes and Haloarenes, students can recall all the concepts quickly.
Haloalkanes and Haloarenes Notes Class 12 Chemistry Chapter 10
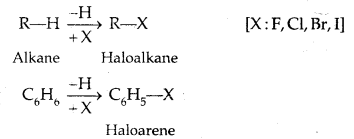
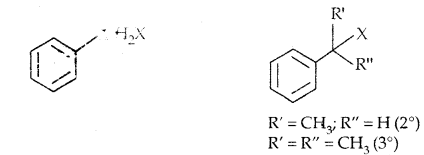
In Haloalkanes X is attached to sp3 hybridized carbon atom, whereas it is attached to sp2 hybridized carbon atom in the aryl group.
Classification:
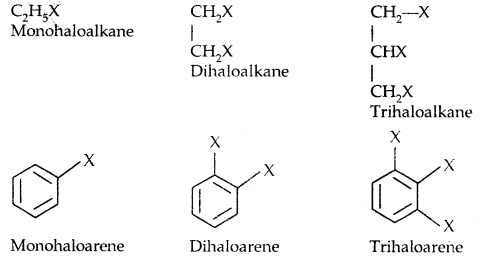
(a) Alkyl halides or Haloalkanes (R-X) [sp3 C-X Bond]
General Formula: Cn H2n-1X
(b) Allylic halides: Here halogen atom is bonded to an sp3-hybridized carbon atom next to C = C, i.e., to an allylic carbon.
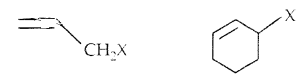
(c) Benzylic halides: Halogen is bonded to sp3 carbon next to the aromatic ring.
Compounds containing sp2 C-X Bond:
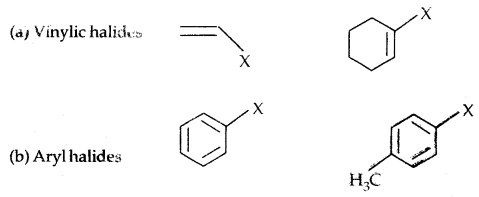
IUPAC name:
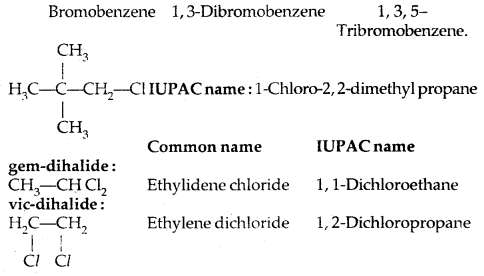
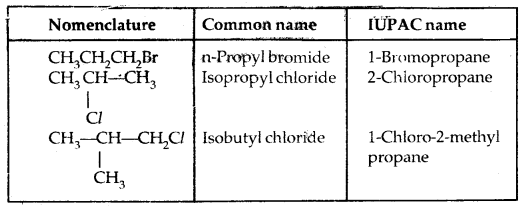
Common and IUPAC names of some halides:
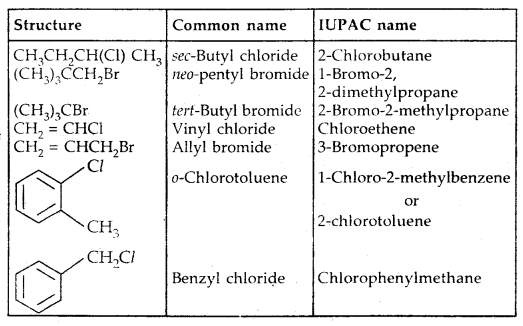
| Structure | Common name | IUPAC name |
| CH2Cl2 | Methylene chloride | Dichloromethane |
| CHCl3 | Chloroform | Trichloromethane |
| CHBr3 | Bromoform | Tribromomethane |
| CCI4 | Carbon tetrachloride | Tetrachl or methane |
| CH3CH2CH2F | n-Propyl fluoride | 1-Fluoropropane |
Nature of C-X bond: Due to the difference in electronegativity of C and X, the C-X bond is polarised; carbon bears a partial positive charge whereas the halogen atom bears a partial negative charge.
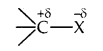
Carbon-halogen bond length increases from C-F to C-I as the size of the halogen atom increases.
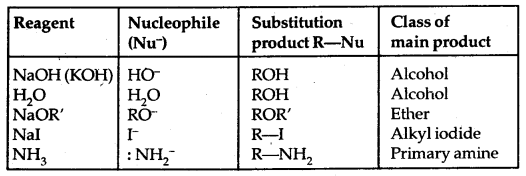
Methods of Preparation:
1. From alcohols
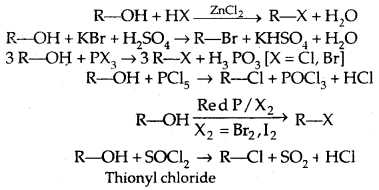
The order of reactivity of alcohols with a given haloacid is 3° > 2° > 1°.
2. From hydrocarbons:
1. By Free radical halogenation: It gives a complex mixture of isomeric mono and polyhaloalkanes which is difficult to separate.
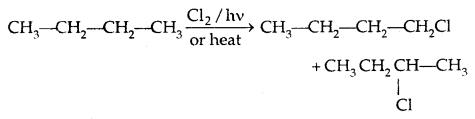
2. By electrophilic Substitution: Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron (III) chloride.
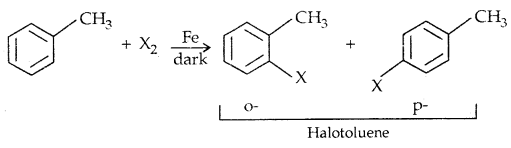
The ortho and para isomers can be easily separated due to large differences in their melting points.
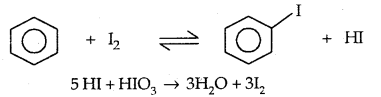
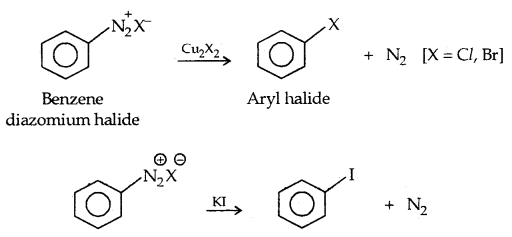
3. Sandmayer’s reaction:
4. From alkenes: (a) Addition of hydrogen halides
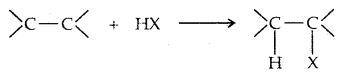
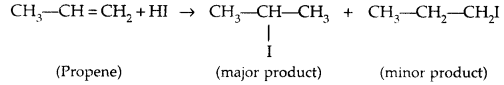
Addition to unsymmetric alkenes is as per Markovnikov’s Rule
(b) Addition of Halogens
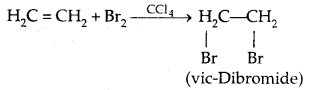
5. Halogen Exchange: Finkelstein Reaction
R-X + Nal → R-I + NaX
X = Cl, Br
Swartz Reaction: This method is used to prepare alkyl fluorides by heating an alkyl chloride/bromide in the presence of AgF/Hg2F2.
H3C-Br + AgF → H3C-F + AgBr
Physical Properties:
- Alkyl halides are colorless when pure. However, bromides and iodides develop color when exposed to light.
- Melting & b.Pts: Lower members are gases at room temperature. Higher members are liquids or solids.
Due to the polar character of the C-X bond and higher molecular mass as compared to the parent hydrocarbon, the intermolecular forces of attraction (dipole-dipole and van der Waals) are stronger in halogen derivatives. That is why boiling points of chlorides, bromides, and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass.
For the same alkyl group, the boiling points increase from RF to RI in the order RF < RCl < RBr < RI.
For isomeric haloalkenes, the b.pts decrease with an increase in branching (lesser the surface area)
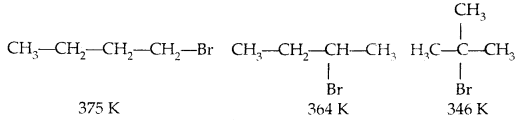
B. pts of isomeric di-halogens are very nearly the same. However, para isomers are higher melting as compared to their ortho and meta isomers. It is due to the symmetry of para isomers that fits in the crystal lattice better as compared to ortho and meta isomers.
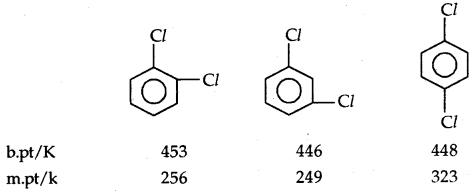
3. Density: Bromo, iodo, and poly-chloro derivatives of hydrocarbons are heavier than water. The density increases with an increase in the number of carbon atoms, halogen atoms, and atomic mass of halogens.
4. Solubility: The haloalkanes are only very slightly soluble in water. However, they tend to dissolve in organic solvents.
Chemical Reactions:
A. Reactions of haloalkanes:
- Nucleophilic substitution reactions (SN)
- Elimination reactions
- Reactions with metals.
1. Nucleophilic Substitution reactions: A nucleophile (Nu– 🙂 reacts with haloalkane (the substrate) which has a polar C-X bond.
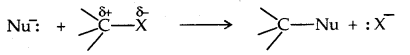
Such reactions in which a stronger nucleophile displaces a weaker nucleophile are called Nucleophilic Substitution (SN) reactions and the halide ion which departs with its bonding pair of electrons is called the leaving group. The better the leaving group, the more facile is the nucleophile substitution reaction. It follows the order
I– > Br > Cl– > F–
∴ The order of reactivity of haloalkanes follows the sequence Iodoalkanes > Bromoalkanes > chloroalkanes > fluoroalkanes.
Types of Nucleophilic Substitution reactions:
- SN2 [Substitution, nucleophilic, bimolecular]
- SN1 [Substitution, nucleophilic, unimolecular]
1. Substitution nucleophilic bimolecular (SN2): The reaction between CH3Cl and hydroxide ion to yield methanol and chloride ion follows second-order kinetics, i.e., the rate depends upon the concentration of both reactants.
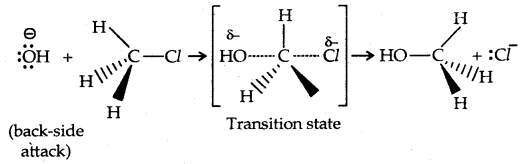
rate of reaction ∝ [Base] [R-X]
Since the rate of the reaction depends upon the concentration of both the reactants, it is a bimolecular nucleophilic displacement reaction.
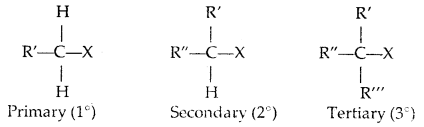
There occurs a complete stereochemical inversion of the configuration. The order of reactivity of the alkyl halides is Primary halide > Secondary halide > Tertiary halide.
In the SN2 reaction, the attack of the nucleophile (OH– above) occurs from the backside, and the halide ion leaves from the front side. This inversion of configuration is called Walden Inversion. As far as the ease of departure of halide ion is concerned, the order of reactivity is RI > RBr > RCl > RF.
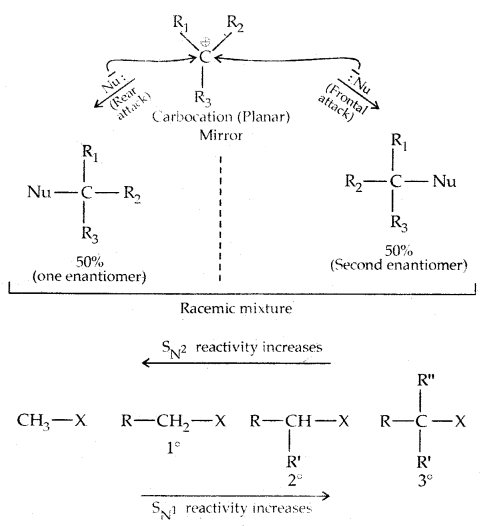
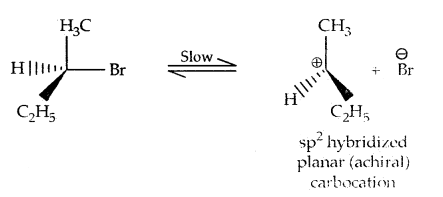
2. Substitution, nucleophilic, unimolecular (SN1) – SN1 reactions are generally carried out in polar protic solvents (like water, alcohol, acetic acid, etc). The reaction between tert-butyl bromide and hydroxide ion yields tert-butyl alcohols and follows first-order kinetics.
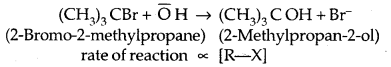
This reaction is independent of the concentration of the base. The rate law suggests the reaction proceeds in two steps.
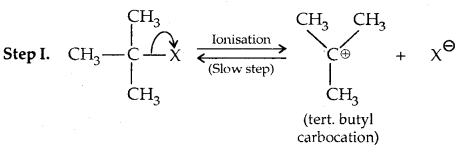
This step is slow and hence is the rate-detaining step.
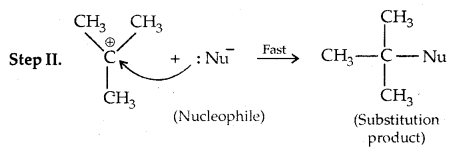
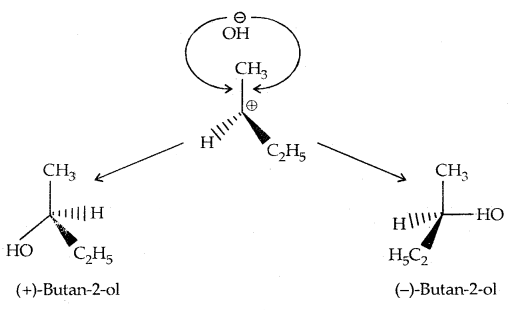
This step, being fast, does not affect the rate of reaction. If the alkyl halide is optically active, then the product is a racemic mixture.
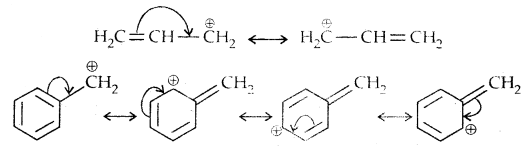
Allylic and benzylic halides show high reactivity towards SN1 reaction
The carbocation gets stabilized through resonance as shown below:
For a given alkyl group, the reactivity of the halide, R-X, follows the same order in both mechanisms.
R-I > R-Br > R-Cl > > R-F.
Stereochemical aspects of nucleophilic substitution reactions: An SN2 reaction proceeds with complete stereochemical inversion of configuration while an SN1 reaction proceeds with racemization.
Optical Activity: Certain compounds exhibit the property of rotating the plane polarised light when it passed through their solutions. Such compounds are called Optically Active compounds arid this phenomenon is called Optical Activity.
If the compound rotates the plane-polarised light to the right, i.e., in a clockwise direction, it is called dextro-rotatory or the d-form and is indicated by placing a positive (+) sign before the degree of rotation. If the light is rotated towards the left (anticlockwise), the compound is said to be laevorotatory or the /-form and a negative sign (-) is placed before the degree of rotation. Such (+) and (-) isomers of a compound are called Optical Isomers and this phenomenon is termed Optical Isomerism.
All the physical properties of compounds showing optical activity are the same like refractive index solubility, density, m.pts, b.pts, etc. Even the extent of rotation is the same. They differ from each other only in the direction of rotation.
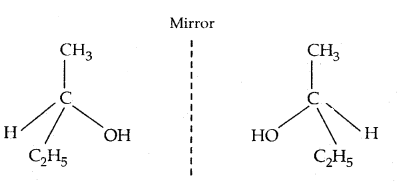
If all the substituents attached to the C atom are different, such a carbon atom is called asymmetric carbon or stereocentre.
The resulting molecule would lack symmetry and is referred to as asymmetric or ‘dissymmetric molecule. This asymmetry of the molecule is responsible for the optical activity in such organic compounds. Such molecules are non-superimposable on their mirror image (as the left hand is non-superimposable on the right hand) and are said to be Chiral. This property of non-super imposibility of the mirror image on the object is called Chirality. The objects which are superimposable on their mirror image are called Achiral.
Butan-2-ol has 4 different groups attached to the tetrahedral carbon atom and is Chiral. The mirror image of Butan-2-ol non-superimposable onbutan-2-ol
Other chiral molecules are Bromochloroiodimethane (BrCl CHI), 2-chlorobutanol, 2, 3-dihydroxypropanal (OHC- CHOH-CH2OH), lactic acid (CH3CH(OH)COOH). The stereoisomers related to each other as non-superimposable are also called Enatiomers and the concept Enantiomerism.
A mixture containing two enantiomers in equal proportions will have zero optical rotation, as the rotation due to one isomer will be canceled by the rotation due to the other isomer. Such a mixture is called Racemic Mixture or Racemic Modification. It is represented by prefixing dl or (±) before the name, e.g., (±) butan-2-ol. The process of conversion of enantiomer into a racemic mixture is known as Racemisation.
Retention: Retention of configuration is the preservation of the integrity of the spatial arrangement of bonds to an asymmetric center during a chemical reaction or transformation. It is also the configurational correlation when a chemical species XCabc is converted into the chemical species YCabc having the same relative configuration.
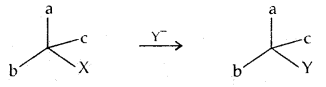
Inversion, retention, and racemization: These are three possibilities for a reaction to occur at an asymmetric carbon atom.
Consider the replacement of a group X and Y in the following reaction:
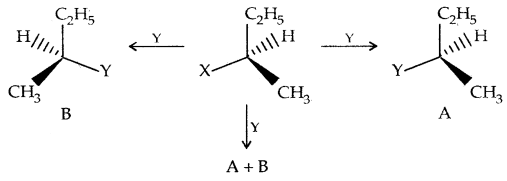
If (A) is the only compound obtained, the process is called retention of configuration.
If (B) is the only compound obtained, the process is called inversion of configuration.
If a 50: 50 mixture of (A) and (B) is obtained, the process is called racemization and the product is optically inactive.
Thus during an SN2 reaction involving an optically active alkyl halide, the reactant undergoes inversion of configuration.
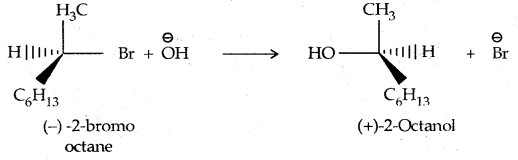
In the case of optically active alkyl halides, SN1 reactions are accompanied by racemization. Consider hydrolysis of optically active 2-romobutane, which results in the formation of(±) butan-2-oI.
Step I:
2. Elimination Reactions: When a haloalkane with a β-hydrogen atom is heated with an alcoholic solution of potassium hydroxide, there is an elimination of hydrogen atom from β-carbon and a halogen atom from the a-carbon atom.
An alkene is formed as a result. Since the β-hydrogen atom is involved in elimination, it is often called β-elimination.
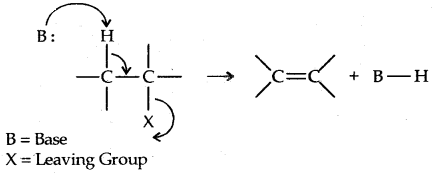
If there is the possibility of the formation of more than one alkene due to the availability of more than one β-hydrogen atom, usually one alkene is formed as the major product. These form part of a pattern first observed by Russian Chemist Alexander Zaitsev (also pronounced as Saytzeff) who in 1875 formulated a rule which can be summarised as in dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms.” Thus, 2-bromopentane gives pent-3-ene as the major product.
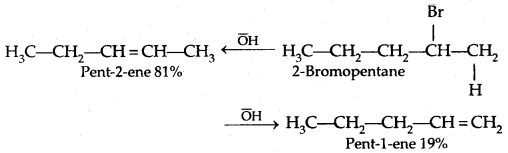
An alkyl halide with β-hydrogen atoms when reacted with a base or a nucleophile has two competing routes: substitution (SN1 and SN2) and elimination. The route to be taken up depends upon the nature of alkyl halide, strength and size of base/nucleophile, and reaction conditions.
Thus, a bulky nucleophile abstracts a proton rather than approaches a tetravalent C atom (steric hindrance). Similarly, a primary alkyl halide will prefer an SN2 reaction, a secondary halide SN2 or elimination depending upon the strength of base/nucleophile, and a tertiary halide: SN1 or elimination depending upon the stability of carbocation or the more substituted alkene.
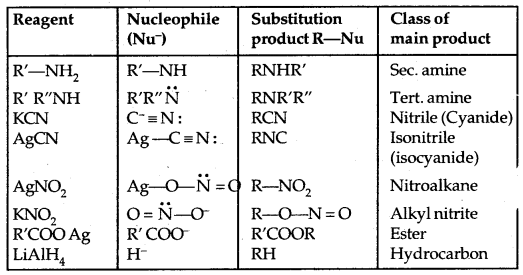
3. Reaction With Metals: Most organic chlorides bromides and iodides react with certain metals to give compounds containing carbon-metal bonds. Such compounds are known as organometallic compounds.
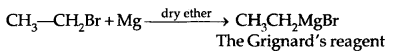
In the Grignard reagent, the C-Mg bond is covalent but highly polar, the MgX bond is essentially ionic.
![]()
The Grignard reagent is highly reactive. Even H2O reacts with it.
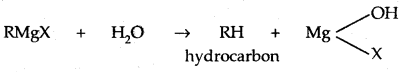
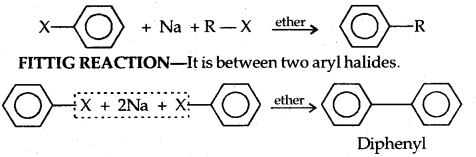
Wurtz Reaction: Alkyl halides react with sodium in dry ether to give hydrocarbons containing double the number of carbon atoms present in the reaction. This reaction is known as the Wurtz reaction.
![]()
Reactions of Haloarenes:
1. Nucleophilic Substitution: Alkyl halides are extremely dull/ loss reactive towards SN reactions due to the following reasons.
1. Resonance effect: In haloarenes, the electron pairs on halogen atom are in conjugation with π-electrons of the ring as given below:
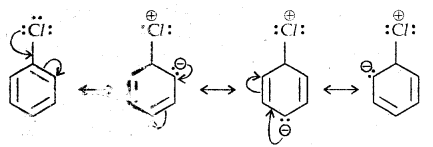
C-Cl bond a partial double bond character due to one. As a result difficult to break the C-X bond and therefore less reactive towards SN1
2. Different hybridization of Carbon atom in C-X bond: In haloalkanes, the C is sp3 hybridized while in haloarenes, the carbon attached to has is sp2 hybridized.
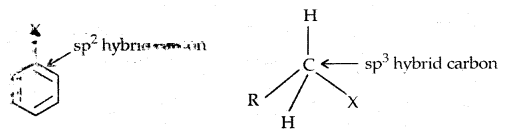
The sp2 hybridized carbon with \(\frac{1}{3}\) s-character is more electronegative and can hold the electron pair of C-X bond more tightly than sp3-hybridized carbon in haloalkane ‘With s-character. Thus
The C-Cl bond length in haloalkane is 177 pm while in haloarene it is 169 pm. Since it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloalkanes towards SN reactions.
3. Instability of phenyl cation: In the case of haloarenes, the phenyl cation formed as a result of self-ionization will not be stabilized by resonance and, therefore, the SN1 mechanism is ruled out.
4. Because of the possible repulsion, it is less likely for the electron-rich nucleophile to approach electron-rich arenes.
Replacement by hydroxyl group:
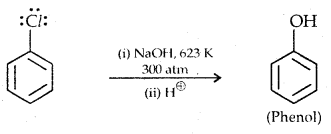
The presence of an electron-withdrawing (-NO2) at ortho and para position increases the reactivity of haloarenes.
The effect is pronounced when the (-NO2) group is introduced at ortho and para positions. However, no effect on the reactivity of haloarenes is observed by the presence of an electron-withdrawing group at meta-position. Mechanism of the reaction is as depicted
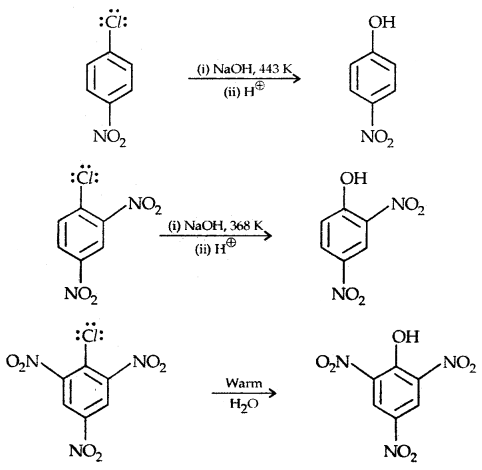
The effect is pronounced when (—NO2 the) group is introduced at ortho and para positions. However, no effect on the reactivity of haloarenes is observed by the presence of an electron-withdrawing group at the meta position. The mechanism of the reaction is as depicted:
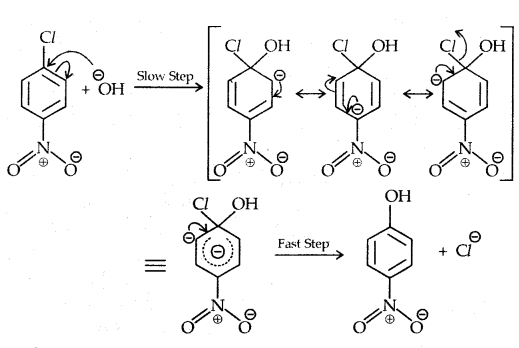
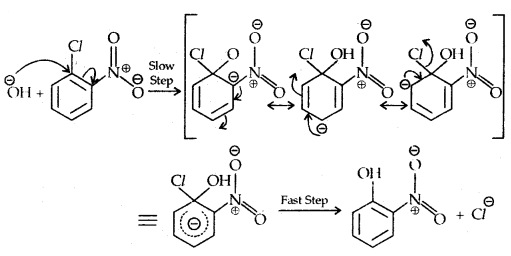
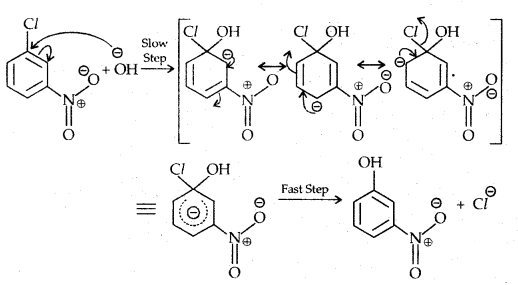
The presence of a nitro group at ortho- and para- position withdraws the electron density from the benzene ring and thus facilitates the attack of the nucleophile on haloarene.
The carbanion thus formed is stabilized through resonance. The negative charge appeared at ortho- and para- positions with respect to the halogen substituent is stabilized by -NO2 group while in the case of mcfa-nitrobenzene, none of the resonating structures bear the negative charge on carbon atom bearing the -NO2 group.
Therefore, the presence of a nitro group of meta-position does not stabilize the negative charge and no effect on reactivity is observed by the presence of the -NO2 group of meta-position.
2. Electrophilic Substitution Reactions: Haloarenes undergo the usual electrophilic reactions of the benzene ring such as halogenation, nitration, sulphonation, and Friedel-Crafts reactions. Halogen atom besides being slightly deactivating is o, p-directing, therefore, further 1 substitution occurs at ortho- and para-positions with respect to the halogen atom.
The o, p-directing; therefore, further substitution occurs at ortho- and para-positions with respect to the halogen atom. The o, p-directing influence of halogen atom can be easily understood if we consider the resonating structures of halobenzene as shown:
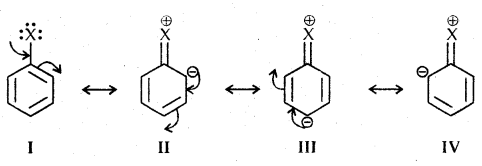
Due to resonance, the electron density increases more at ortho- and para-positions than at mcia-positions. Further, the halogen atom because of its-I effect has some tendency to withdraw electrons from the benzene ring. As a result, the ring gets somewhat deactivated as compared to benzene and hence the electrophilic substitution reactions in haloarenes occur slowly and require more drastic conditions as compared to those in benzene.
1. Halogenation:
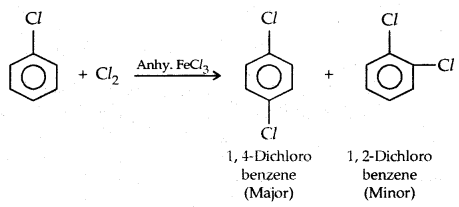
2. Nitration:
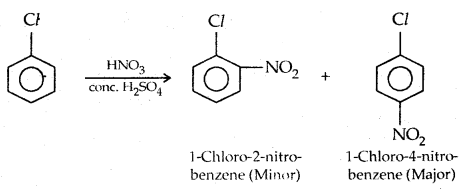
3. Sulphonation:
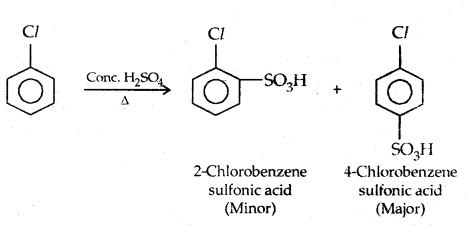
4. Friedel-Crafts reaction:
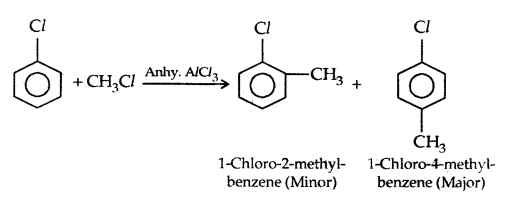
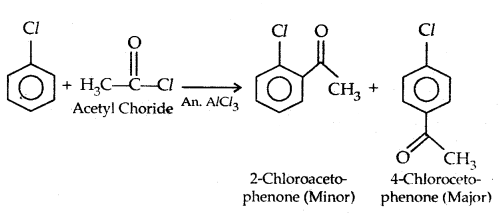
3. Reaction with metal:
Wurtz-Fitting Reaction: It is between an alkyl and aryl halide.
→ Polyhalogen Compounds: Carbon compounds containing more than one halogen atom are usually referred to as polyhalogen compounds.
→ Dichloromethane (Methylene chloride CH2Cl3: It is widely used as a solvent in paint remover, as a propellant in aerosols, and as a process solvent in the manufacturing of drugs. It harms the human central nervous system.
→ Trichloromethane (Chloroform CHCl3): It is used as a solvent for fats, alkaloids, iodine, waxes, rubbers, plastics, etc. It was used as a general anesthetic in surgery.
Chloroform is slowly oxidized to carbonyl chloride (phosgene) by air in the presence of light. It is extremely poisonous in nature.
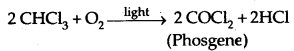
It is therefore stored in colored bottles to cut off light and in well- stoppered fully filled bottles to cut off air.
→ Triiodomethane (Iodoform CHI33): It was used as an antiseptic, but the antiseptic properties are due to the liberation of free iodine and not due to the iodoform itself.
→ Tetrachloromethane (Carbon Tetrachloride CCl4): It is used for the synthesis of chlorofluorocarbons and as a solvent. There is some evidence that exposure to CCl4 causes liver cancer in humans. It causes dizziness, nausea, and vomiting which can cause permanent damage to nerve cells. The chemical may irritate the eyes on contact. It depletes the ozone layer when released into the air.
→ Freons: Chlorofluorocarbon compounds of methane and ethane are collectively known as freons. They are extremely stable, unreactive, non-toxic, non-corrosive, and easily liquefiable gases, Freon 12 (CCl2F2) is one of the most common freons in industrial use. Most freon, even that used in refrigeration, eventually makes its way into the atmosphere where it diffuses into the stratosphere where it is able to imitate radical change reactions that can upset the natural ozone balance.
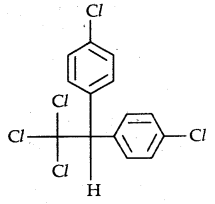
→ p, p’-Dichlorodiphenyltrichloroethane (DDT): The use of DDT was effectively used against the mosquito that spreads malaria and lice that carry typhus. Many species of insects developed resistance to DDT, and DDT was also discovered to have a high degree of toxicity towards fish. The chemical stability of DDT and its fat solubility compounded the problem. The use of DDT was banned in the USA in 1973, although it is still in use in some parts of the world. Its chemical formula is
Many out of these polyhalogen compounds cannot be easily decomposed and cause of depletion of the ozone layer and are proving environmental hazards.