By going through these CBSE Class 12 Chemistry Notes Chapter 11 Alcohols, Phenols and Ethers, students can recall all the concepts quickly.
Alcohols, Phenols and Ethers Notes Class 12 Chemistry Chapter 11
→ Alcohol contains one or more hydroxyl (OH) group (s) directly attached to a carbon atom (s).
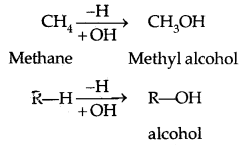
→ A Phehol contains one or more hydroxyl group (OH) attached to a carbon atom (s) of the benzene ring.
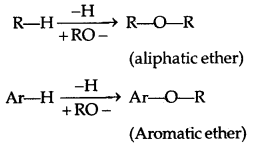
→ An ether contains an alkoxy/aryloxy group (R-O/Ar-O) in place of the H atom of a hydrocarbon.
Classification:
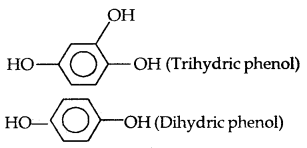
- C2H5OH: Monhydric alcohol
- CH2OH-CH2OH: Dihydric alcohol
- HOH2C-CHOH-CH2OH: Trihydric alcohol

1. Compounds containing Csp3 -OH bond: In this class of alcohols, the -OH group is attached to an sp3 hybridized carbon atom of an alkyl group. They are further classified as follows:
→ Primary, secondary and tertiary alcohols: Here -the OH group is attached to a primary, secondary and tertiary carbon atom, respectively as shown below:
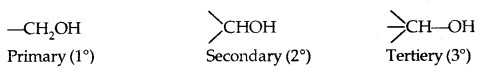
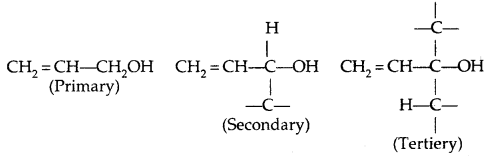
→ Allylic alcohols: In these alcohols, -OH group is attached to an sp3 hybridized carbon next to the carbon-carbon double bond, i.e., to an allylic carbon, e.g.,
→ Benzylic alcohols: In these alcohols, the -OH group is attached to an sp3-hybridized carbon next to an aromatic ring. For example
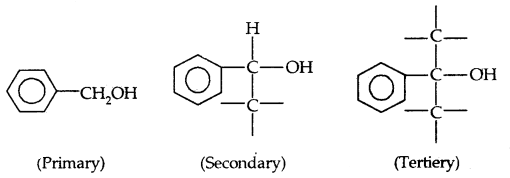
Allylic and benzylic alcohols may be primary, secondary or tertiary.
2. Compounds containing Csp2-OH bond: These alcohols contain -OH group bonded to a carbon-carbon double bond.
Vinylic alcohols CH2 = CH-OH
Ethers (A) Simple ethers/Symmetrical ethers (ROR)-
C2H5-O-C2H5 CH3-O-CH3.
(B) Mixed or Unsymmetrical ether (ROR’): R ≠ R’
C2H-O-CH3 C6H5-O-C2H5
Nomenclature:
(a) Alcohols: Common and I.U.P.A.C. names given below:
Table 11.1: Common and IUPAC Names of Some Alcohols:
Cyclic alcohols are named using the prefix cyclo and considering the-OH group attached to C-1.
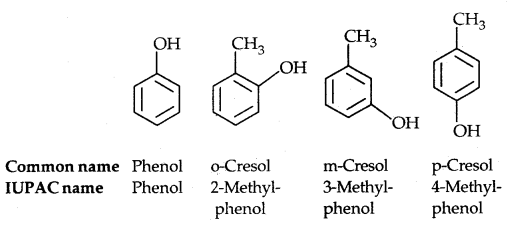
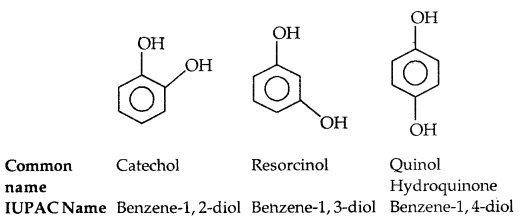
(b) Phenols: The simplest hydroxy derivative of benzene is phenol, it is its common name and also an accepted IUPAC name. As the structure of phenol involves a benzene ring, in its substituted compounds the terms ortho (1, 2- disubstituted), meta (1, 3-disubstituted) and para (1, 4-disubstituted) are often used in the common names.
Dihydroxy derivatives of benzene are known as 1, 2-, 1, 3- and 1, 4-benzenediol.
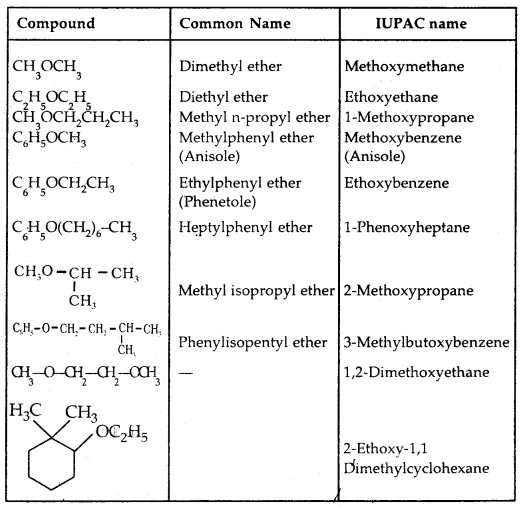
(c) Ethers: Common names of others are derived from the names of alkyl/aryl groups written as separate words in alphabetical order and adding the word ‘ether’ at the end. For example, CH3OC2H5 is ethyl methyl ether. If both the alkyl groups are the same, the prefix ‘di’ is added before the alkyl group. For example, C2H5OC2H5 is diethyl ether.
According to the IUPAC system of nomenclature, others are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an -OR or -OAr group, where R and Ar represent alkyl and aryl groups, respectively. The larger (R) group being chosen as the parent hydrocarbon. The names of a few others are given as examples in the Table below:
Table 11.2: Common and IUPAC Names of Some Ethers:
Structures of Functional Groups-In alcohols, the oxygen of the -OH group is attached by a sigma (a) bond formed by the overlap of an sp3 hybridized orbital of carbon with an sp3 hybridized orbital of oxygen.
The figure below depicts the structural aspects of the methanol, phenol and methoxymethane.
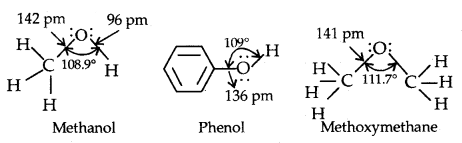
Structures of methanol, phenol and methoxymethane.
The bond angle in alcohols is slightly less than the tetrahedral angle (109°28′) which is due to repulsion between the unshared electron pairs of oxygen.
Alcohols and Phenols:
Preparation of Alcohols:
1. From alkenes:
1. By acid-catalysed hydration
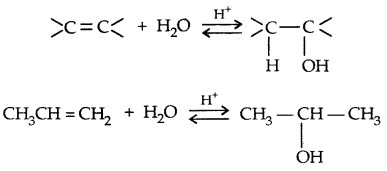
Mechanism:
→ Step I: Protonation of alkene to form carbocation by the electrophilic attack of H3O+ ion,
H2O + H+ → H3O+
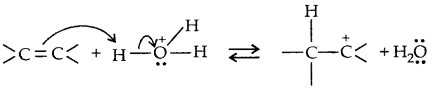
→ Step II: Nucleophilic attack of water on carbocation
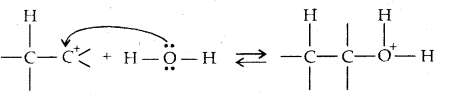
→ Step III: Deprotonation to form an alcohol
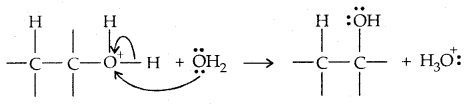
3. By hydroboration-oxidation: Borane (BH3) is an electrophile since it is electron-deficient. Addition product formed is oxidized to alcohols by H2O3 and aq. NaOH.
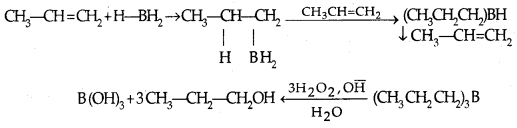
Addition of water proceeds against the Markovnikor rule.
2. From Carbonyl Compounds:
1. Reduction of aldehydes and Ketones:
(a) Hydrogen gets added in the presence of a catalyst (catalytical hydrogenation) like Pd, Pt, Ni (all finely. divided)
(b) By treating carbonyl compounds with sodium borohydride or lithium aluminium hydride (Li A1H4).
Aldehydes give primary alcohols whereas ketones yield secondary alcohols.
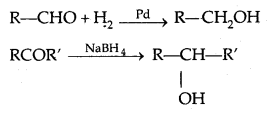
2. Reduction of carboxylic acids and esters:
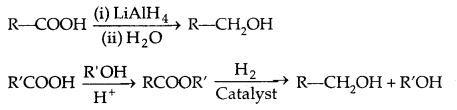
3. From Gngnard reagents:
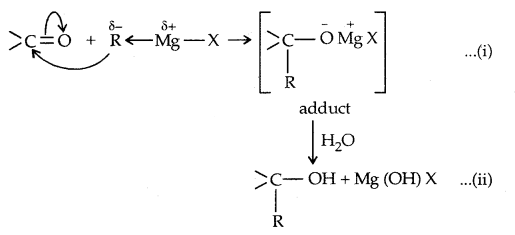
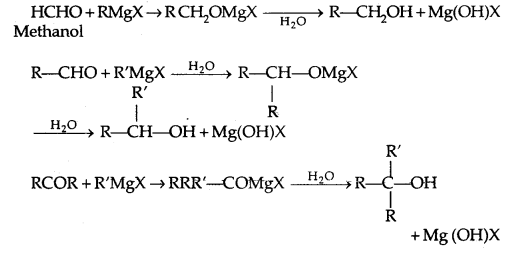
Preparation of Phenols:
1. From haloarenes
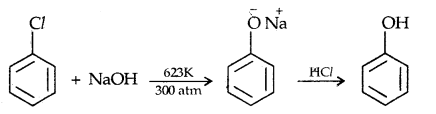
2. From benzene Suiphonic acid
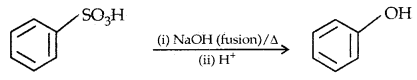
3. From diazonium salts
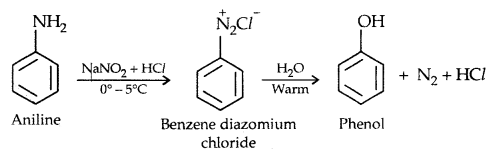
4. From Cumene
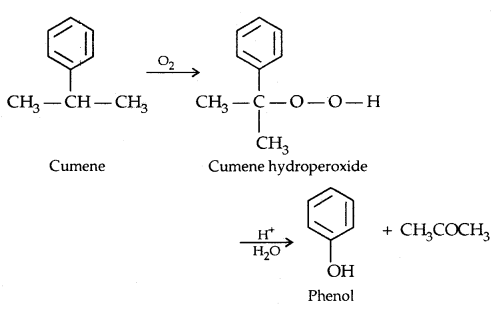
→ Physical Properties of Alcohols and Phenols: The properties of alcohols and phenols are chiefly due to the hydroxyl group. The nature of alkyl and aryl groups simply modify their properties.
→ Boiling Points: The boiling points of alcohols and phenols increase With the increase in the number of carbon atoms (increase in van der Waals forces). In alcohols, the boiling points decrease with the increase of branching in the carbon chain (because of a decrease in van der Waals forces due to a decrease in surface area).
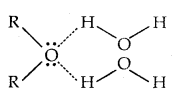
The -OH group is alcohols and phenols is involved in intermolecular hydrogen bonding as shown below:
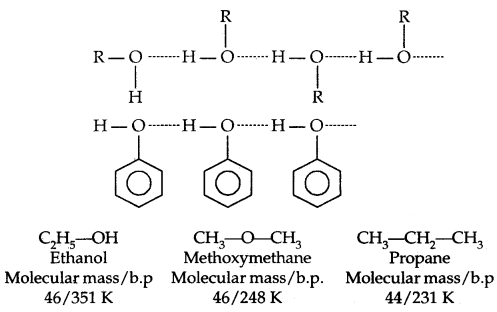
The high boiling points of alcohols are mainly due to the presence of intermolecular hydrogen bonding in them which is lacking in ethers and hydrocarbons.
→ Solubility: Solubility of alcohols and phenols in water is due to their ability to form hydrogen bonds with water molecules. The solubility decreases with an increase in the size of alkyl/aryl (hydrophobic) groups. Lower alcohols are miscible with water in all proportions.
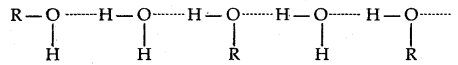
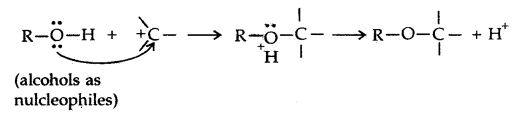
→ Chemical Reactions: Alcohols react both with nucleophiles and electrophiles. The bond between O-H is broken when alcohols react as nucleophiles.
1. 
2. The bond between C-O is broken when they react as electrophiles. Protonated alcohols react in this manner.
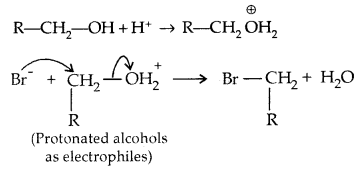
Based on the cleavage of O-H and C-O bonds, the reactions of alcohols and phenols may be divided into two groups.
(a) Reactions involving cleavage of O-H bond:
1. Acidity of alcohols and phenols
→ Reactions with metals:
2R-O-H + 2 Na → 2 RONa + H2
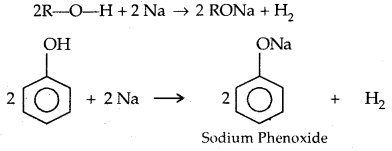
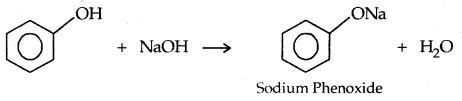
→ Reaction with Aq. NaOH: Phenols react with aq. NaOH
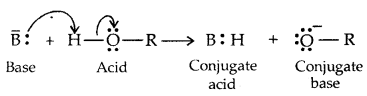
→ Alcohols and Phenols are Bronsted acids i.e., they can donate a proton to a stronger base [: B]
→ The acidity of alcohols:
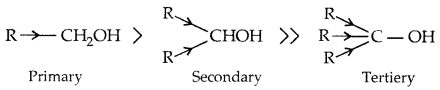
Due to the electron-releasing group (-CH3, -C2H5) electron density on oxygen increases and the polarity of the O-H bond decreases. This decreases the acid strength.
Alcohols are, however, weaker acids than water.
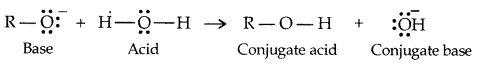
Water is a better proton donor (i.e., stronger acid) than alcohol. Alkoxides (e.g. sodium ethoxide) is a better proton-acceptor than hydroxide ion, which suggests that alkoxides are stronger bases. (Sodium ethoxide is a stronger base than sod. hydroxide).
Alcohols act as Bronsted bases as well. It is due to the presence of unshared electron pairs on oxygen, which makes them proton acceptors.
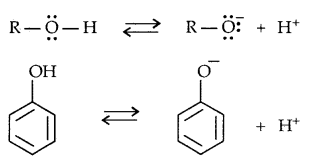
→ The acidity of Phenols: The reactions of phenols with metals like Na, Al and NaOH indicate their acidic nature. The -OH group in phenols is directly attached to the sp2 hybridized carbon of benzene ring which acts as an electron-withdrawing group.
The reaction of phenol with aqueous sodium hydroxide indicates that phenols are stronger acids than alcohols and water. Let us examine how a compound in which the hydroxyl group attached to an aromatic ring is more acidic than the one in which the hydroxy] group is attached to an alkyl group.
The ionisation of alcohol and a phenol takes place as follows:
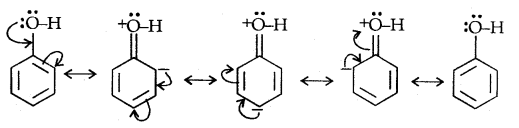
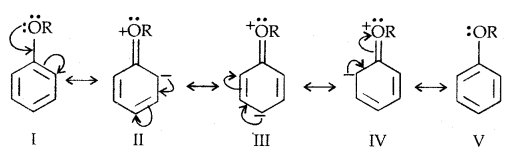
Due to the higher electronegativity of sp2 hybridised carbon of phenol to which -OH is attached, electron density decreases on oxygen. This increases the polarity of the O-H bond and results in an increase in ionisation of phenols than that of alcohols. Now let us examine the stabilities of alkoxide and phenoxide ions. In alkoxide ion, the negative charge is localised on oxygen while in phenoxide ions, the charge is delocalised.
The delocalisation of negative charge (structures I-V) makes phenoxide ion more stable and favours the ionisation of phenol. Although there is also charge delocalisation in phenol its resonance structures have charge separation due to which the phenol molecule is less stable than phenoxide ion.
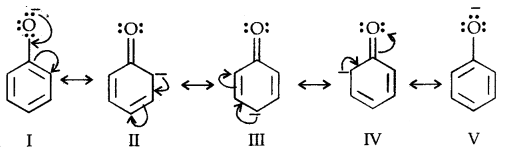
In substituted phenols, the presence of electron-withdrawing groups such as the nitro group enhances the acidic strength of phenol. This effect is more pronounced when such a group is present at ortho and para positions. It is due to the effective delocalisation of negative charge in phenoxide ion.
On the other hand, electron releasing groups, such as alkyl groups, in general, do not favour the formation of phenoxide ion resulting in a decrease in acid strength. Cresols, for example, are less acidic than phenol.
Table 11.3: pKa Values of Some Phenols and Ethanol:
| Compound | Formula | pKa |
| o-Nitrophenol | 0-O2N-C6H4-OH | 7.2 |
| m-Nitrophenol | m-O2N-C6H4-0H | 8.3 |
| p-Nitrophenol | p-O2N-C6H4-0H | 7.1 |
| Phenol | C6H5-OH | 10.0 |
| o-Cresol | O-CH3-C6H4-OH | 10.2 |
| m-Cresol | m-CH3C6H4-OH | 10.1 |
| p-Cresol | p-CH3-C6H4-OH | 10.2 |
| Ethanol | C2H5OH | 15.9 |
From the above data, you will note that phenol is a million times more acidic than ethanol.
2. Esterification: Alcohols and phenols react with carboxylic acids, acid chlorides and acid anhydrides to form esters.
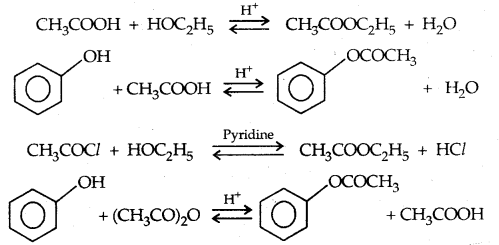
The introduction of acetyl (CH3 CO–) group in alcohols or phenols is known as acetylation. Acetylation of salicylic acid produces aspirin which possesses analgesic, anti-inflammatory and antipyretic properties.
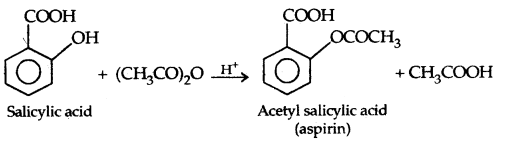
(b) Reactions involving cleavage of C-O bond in alcohols.
1. Reaction with HX:
ROH + HX → R-X + H2O.
Luca’s Test distinguishes the three classes of alcohols (1°, 2° and 3°) on reaction with cone. HCl and ZnCl2 [Luca’s reagent], 3° alcohols produce turbidity immediately with it. 2° alcohols do it after some time. 1° alcohol does not produce turbidity at room temperatures.
2. Reactions with phosphorus trihalides:
3R-OH + PCl3 → 3R-Cl + H3P03
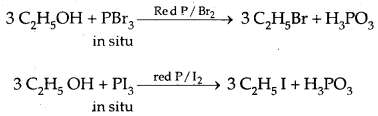
3. Reaction with a protic acid: e.g., cone. H3P04 or H2SO4 causes dehydration of alcohols producing alkenes.
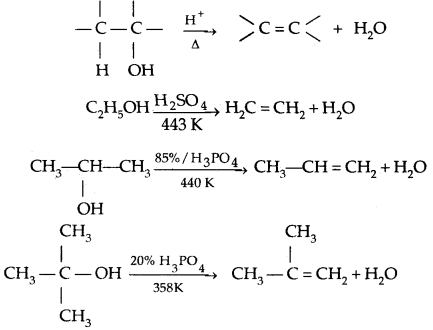
Thus the relative ease of dehydration of alcohols follows the order Tertiary > Secondary > Primary
Mechanism of dehydration of alcohols:
→ Step I: Formation of protonated alcohol
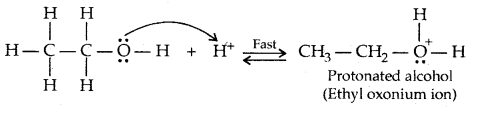
→ Step II: Formation of the carbocation. It is the slow step and hence the rate-determining step.
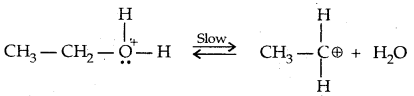
→ Step III: Formation of ethene by loss of a proton.
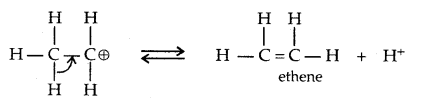
4. Oxidation:
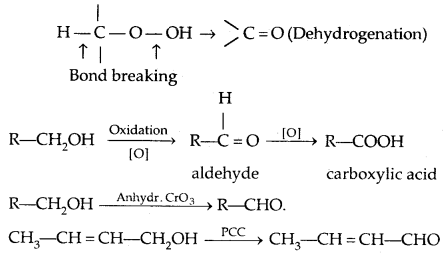
(PCC is pyridinium chlorochromate-a complex of chromium oxide with pyridine and HCl)
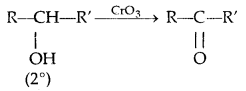
Tertiary alcohols do not undergo oxidation reactions.
5. Dehydrogenation with red hot copper: I° and 2c alcohol form aldehydes and ketones respectively. 3° alcohols undergo dehydration.
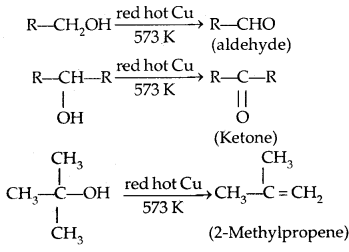
(c) Reactions of Phenols:
Following reactions are shown by phenols only.
1. Electrophilic aromatic substitution: The —OH group fused in the ring activates the ring towards electrophilic substitution directing the incoming group at ortho and para positions.
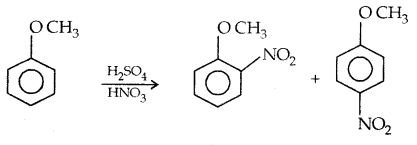
1. Nitration:
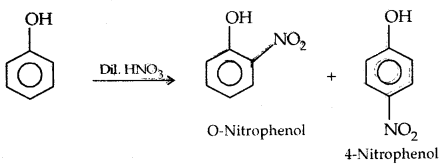
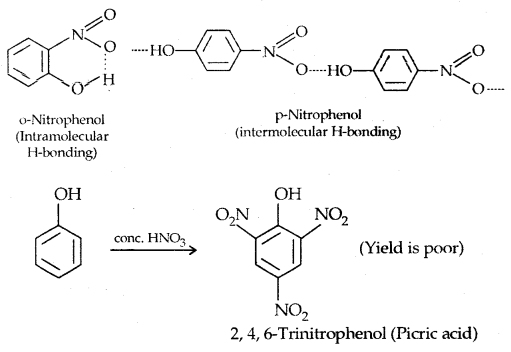
The o- and p-isomers can be separated by steam distillation, o-, Nitrophenol is steam volatile due to intramolecular H-bonding while p-nitrophenol (Higher B.Pt) is less volatile due to intermolecular H- bonding which causes the association molecules.
2. Halogenation:
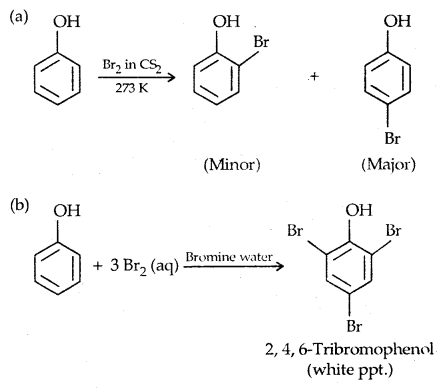
Reaction with Bromine water is used as a test of phenol.
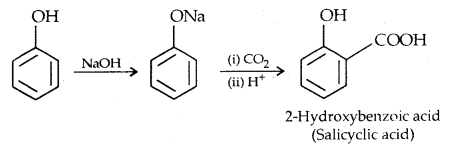
2. Koibe’s reaction:
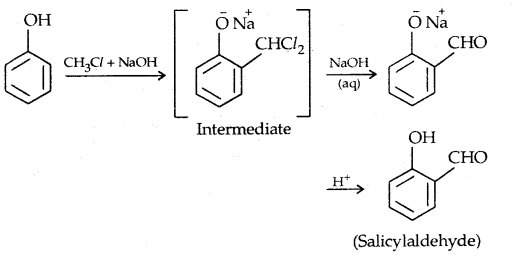
3. Reimer-Tiemann reaction: Salicylaldehyde is formed.
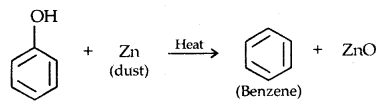
4. Reaction with zinc dust: Benzene is formed.
5. Oxidation:
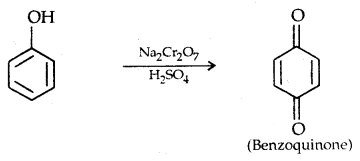
Some Commercially Important Alcohols:
1. Methanol (CH3OH): It is also known as wood-spirit.
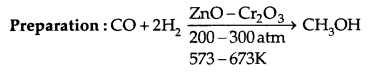
Properties: It is a colourless liquid. It boils at 337 K. It is highly poisonous in nature. Ingestion of even small quantities causes blindness and large quantities cause even death.
Uses:
- It is used as a solvent in paints, varnishes etc.
- It is chiefly used for making formaldehyde.
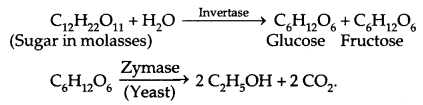
2. Ethanol (C2H5OH):
Commercial Preparation: By fermentation of molasses
Properties:
- It is a colourless liquid with B.Pt 351 K.
- Combined with CuSO4 and pyridine, it is termed as denatured spirit
Uses:
- It is an excellent solvent.
- In the laboratory and hospitals for sterilisation of surgical instruments.
Ethers:
Preparation of Ethers:
1. By dehydration of alcohols:
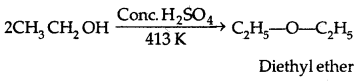
It is a nucleophilic bimolecular reaction (SN2)
Step:
![]()
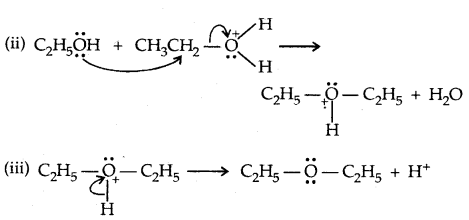
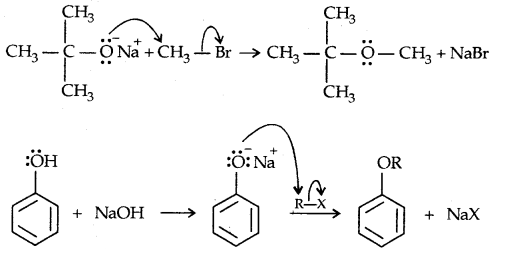
2. Willamson synthesis of ethers: It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers.
![]()
It involves the SN2 attack of an alkoxide ion on primary alkyl halide.
Physical Properties of ethers:
- The C-O bonds in ethers are polar and thus ethers have a net dipole moment.
- Their b. pts are comparable to those of alkanes with comparable molecular masses but lower than those of alcohols. It is due to the lack of H-bonding in ethers.
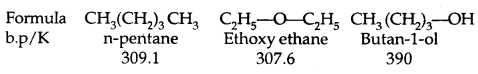
- The miscibility of ethers with water resembles those of alcohols of the same molar mass. It is due to the fact that-ethers like alcohols can form H-bonds with water.
Chemical Reactions: Ethers are the least reactive of the functional groups.
1. Cleavage of C-O bond in ethers-lire the cleavage of C-O bond in ethers takes place under drastic conditions with an excess of hydrogen halides.
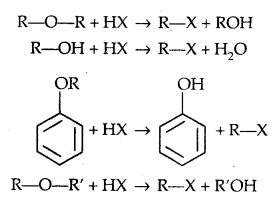
The order of reactivity is HI > HBr > HCl.
Mechanism:
→ Step I:
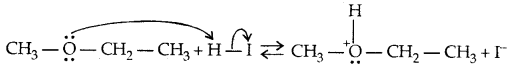
→ Step II:
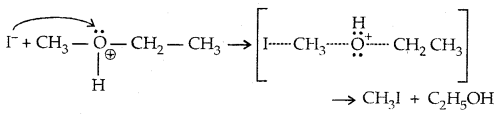
With HI in excess and at a high temp., ethanol reacts with another molecule of HI and is converted to ethyl iodide.
→ Step III:
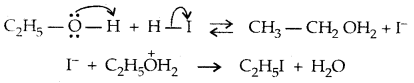
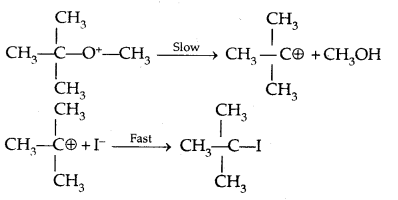
However, when one of the alkyl groups is tertiary, the halide formed is a tertiary halide.
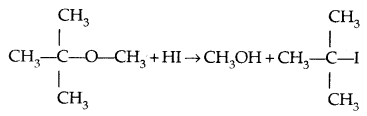
It is because, in step-2 of the reaction, the departure of leaving group (HO- CH3) creates a more stable carbocation [(CH3)3 C+] and the reaction follows the SN1 mechanism.
2. Electrophilic substitution: The alkoxy group (-OR) is ortho and para directing and activates the aromatic ring towards electrophilic substitution.
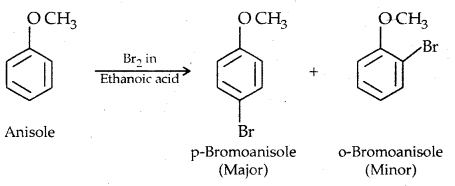
1. Halogenation: Phenylalkyl ethers undergo usual halogenation in the benzene ring, e.g., anisole undergoes bromination with bromine in ethanoic acid even in absence of iron (III) bromide catalyst. It is due to the activation of the benzene ring by the methoxy group. Para isomer is obtained in 90% yield.
2. Friedel Crafts reaction: Anisole undergoes Friedel Crafts reaction, i.e., the alkyl and acyl groups are introduced at ortho and para positions by reaction with an alkyl halide and acyl halide in the presence of anhydrous aluminium chloride (a Lewis acid) as a catalyst.
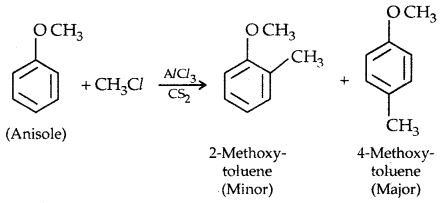
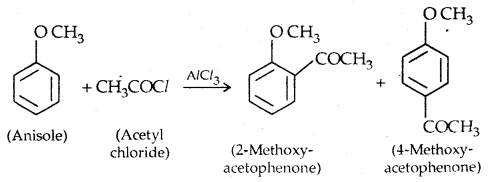
3. Nitration: Anisole reacts with a mixture of the cone. H2SO4 and HNO3 to yield a mixture of ortho and para nitro anisole.