By going through these CBSE Class 12 Chemistry Notes Chapter 13 Amines, students can recall all the concepts quickly.
Amines Notes Class 12 Chemistry Chapter 13
Amines: Amines can be considered as derivatives of ammonia, obtained by replacement of H of NH3 by alkyl /aryl group.
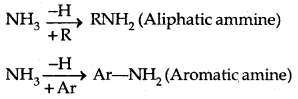
→ Structure of Amines: Like ammonia, the N atom of amines is trivalent and Carnes an unshared pair of electrons. N orbitals in amines are sp3 hybridized and the geometry of amines is pyramidal.
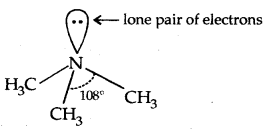
(Pyramidal shape of trimethylamine)
→ Classification of Amines: Amines are classified as primary (1° secondary (2°) and tertiary (3°) depending upon the no. of hydrogen atoms replaced by alkyl/aryl groups in ammonia molecule.
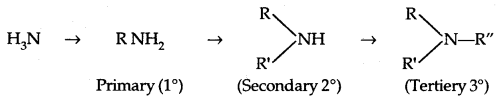
Amines are simple if R = R’ = R”
They are termed as mixed when R, R’, R” are different.
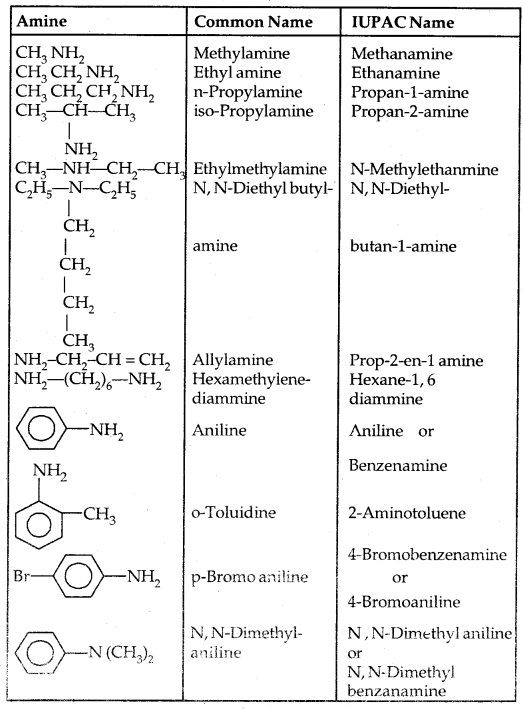
→ Nomenclature of Amines: In the IUPAC system ‘e’ of the alkane is replaced by ‘amine’ like Alkanamine. The simplest arylamine is aniline (C6H5—NH2) or benzene amine
→ Preparation of Amines:
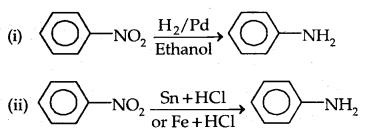
1. Reduction of Nitro Compounds
2. Ammonolysis of Alkyl Halides
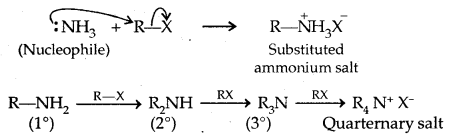
The order of reactivity of halides is RI > RBr > RCI.
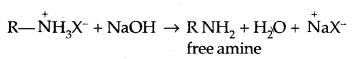
3. Reduction of Nitriles
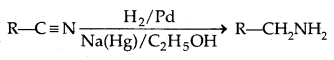
4. Reduction of amides
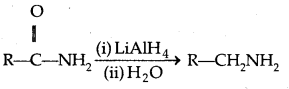
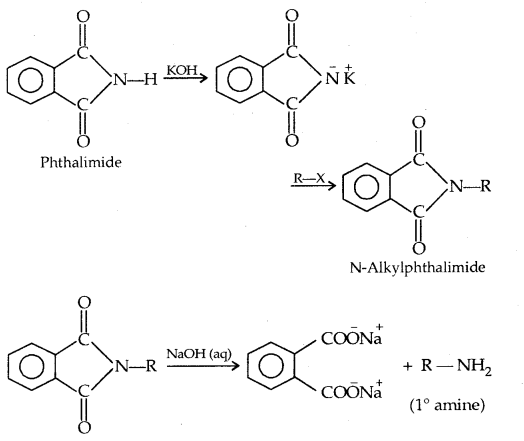
5. Gabriel Phthalimide Synthesis: This method is used to prepare pure primary amines. Aromatic amines cannot be prepared by this method.
Hoffman Bromamide Degradation Reaction: Primary amines are obtained when an amide is treated with Br2 in an aqueous solution of NaOH.
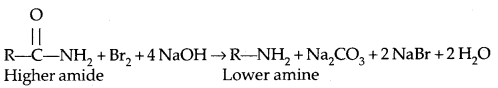
Physical Properties:
- Lower aliphatic amines are gases with a fishy odour. Primary amines with three or more C atoms are liquids and still higher ones are solids.
- Lower aliphatic amines are soluble in water because they can form H-bonds with water molecules. Higher amines are essentially insoluble in water.
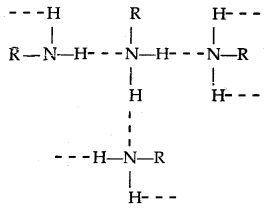
- The order of boiling points of isomeric amines is Primary > Secondary > Tertiary. It is due to the presence of intermolecular H-bonding which is more in primary amines (due to the presence of two H- atoms attached to N than only one in secondary amines), less in 2° amines and absence in tertiary amines.
Chemical Reactions:
1. Basic character of Amines: Amines being basic in character (Lewis bases) react with acids to form salts.
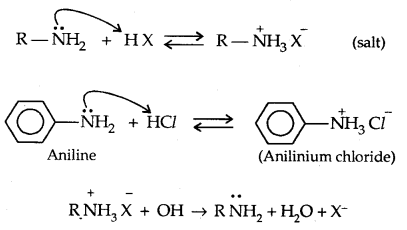
The basic character of amines can be better understood in terms of their PKb and kb values as explained below:
![]()
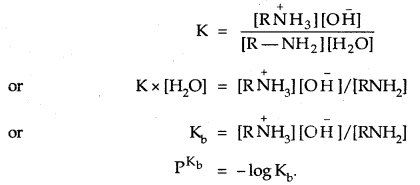
The larger the value of Kb or lower the value of PKb, the stronger is the base. PKb value of ammonia is 4.75. Aliphatic amines are stronger bases than ammonia due to the + I-effect of alkyl groups. The availability of lone pair of electrons on N increases. On the other hand, aromatic amines are weaker bases than ammonia due to the electron-withdrawing nature of the aryl group. The PKb values of few amines are given below:
Table: PKb values of Amines in Aqueous Phase:
| Name of amine | PKb |
| Methenamine | 3.38 |
| N-Methylmethanamine | 3.27 |
| N, N-Dimethylmethanamine | 4.22 |
| Ethanamine | 3.29 |
| N-Ehtylethamine | 3.25 |
| Benzenamine | 9.38 |
| Phenylmethanamine | 4.70 |
| N-Methylaniline | 9.30 |
| N, N-Dimethylaniline | 8.92 |
Besides the + I or – I effect, the interplay of other factors like solvation effect, steric hindrance etc. affect the basic strength of amines.
→ Structure-Basicity Relationship of Amines: The basicity of amines is related to their structure. The basic character of an amine depends upon the ease of formation of the cation by accepting a proton from the acid.
The more stable the cation is relative to the amine, the more basic is the amine.
(a) Alkamines versus Ammonia
Let us consider the reaction of an alkanamine and ammonia with a proton to compare their basicity.
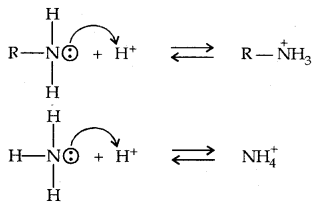
Due to the electron releasing nature of the alkyl group, it (R) pushes electrons towards nitrogen and thus makes the unshared electron pair more available for sharing with the proton. Moreover, the substituted ammonium ion formed from the amine gets stabilised due to dispersal of the positive charge by the +1 effect of the alkyl group. Hence, alkylamines are stronger bases than ammonia.
Thus the basic nature of aliphatic amines should increase with the increase in the number of alkyl groups. This trend is followed in the gaseous, phase. The order of basicity of amines in the gaseous phase follows the expected order: tertiary amine > secondary amine > primary amine > NH3.
The trend is not regular in the aqueous state as evident by their PKb values given in Table. In the aqueous phase, the substituted ammonium cations get stabilised not only by the electron releasing effect of the alkyl group (+1) but also by hydrogen bonds and solvation with water molecules. The greater the size of the ion, the lesser will be the solvation and the less stabilised the ion. The order of stability of ions are as follows:
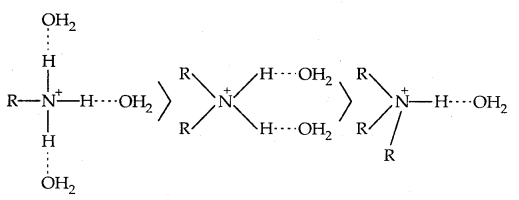
Decreasing order of extent of H-bonding in water and stabilization by solvation: Greater the stability of the substituted ammonium cation, the stronger base is the corresponding amine. Thus the order of basicity of aliphatic amines should be primary > secondary > tertiary, which is opposite to the inductive effect based order. Secondly, when the alkyl group is small like – CH3 group, there is no steric hindrance to H-bonding.
In case the alkyl group is bigger than the CH3 group, there will be some steric hindrance to H-bonding. Therefore, the change of nature of the alkyl group, e.g., from CH3 to – C2H5 results in a change in the order of basic strength. Thus, there is a subtle interplay of the inductive effect, solvation effect and steric hindrance of the alkyl group which decides the basic strength of alkylamines in the aqueous state.
The order of basic strength in the case of methyl-substituted amines and ethyl substituted amines in an aqueous solution is as follows:
(C2H5)2 NH > (C2H5)3N > C2H5NH2 > NH3
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
(b) Arylamine Vs Ammonia: High value of PKb of aniline suggests that it is a much weaker base than aliphatic amines or even ammonia. It is due to the resonance shown by aniline.
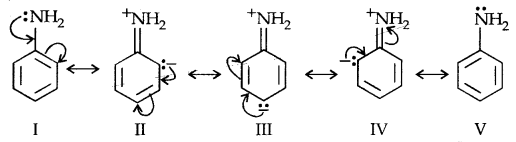
Aniline is a resonance hybrid of 5 canonical structures out of which there is no lone pair of electrons available for sharing on N in II, III and IV structures. It results in the unshared electron pair on N being in conjugation with the benzene ring making it less available for protonation.
On the other hand, aclidinium ion obtained by accepting a proton can have only two resonating structures (Kekule) as shown below:
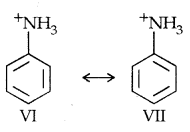
The greater the no, of resonating structures, the greater is the stability. Thus aniline is more resonance stabilized than anilinium ion. Hence the proton acceptability or the basic nature of aniline and other aromatic amines would be less than ammonia.
In the case of substituted aniline, electron-releasing groups like – OCH3 – CH3 increase the basic strength whereas electron-withdrawing groups like – NO2, – SO3H, – COOH, – X decrease it.
Thus (C2H5)2 NH > C2H5NH2 > NH3 > C6H5NH2
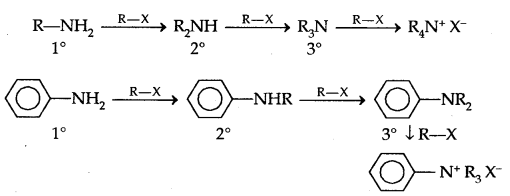
2. Alkylation: Amines undergo alkylation with alkyl halides.
3. Acylation: Aliphatic and aromatic primary and secondary amines react with acid chlorides, anhydrides and esters by a nucleophilic substitution reaction. This reaction is known as acylation.
It is the replacement of H of – NH2 group
or
![]()
group by the acyl group.
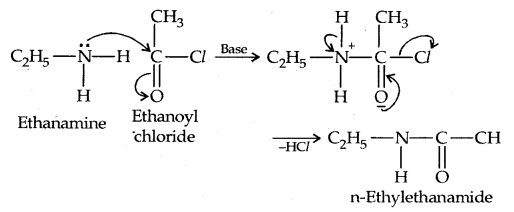
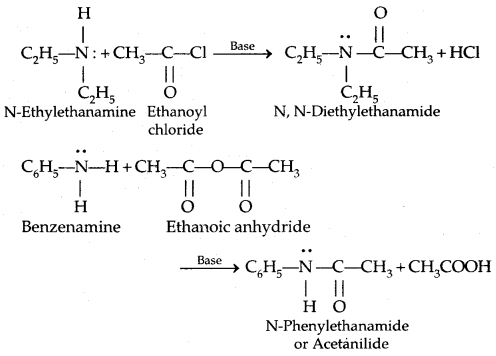
The reaction of amines with benzoyl chloride (CH5 COCl) is called Benzoylation.
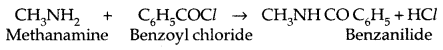
4. Carbylamine reaction: It is a test of primary amines-both aliphatic and aromatic.
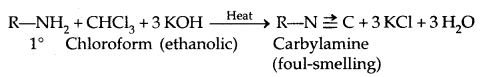
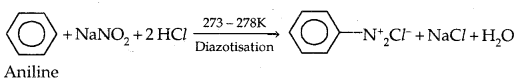
5. Reaction with Nitrous acid: All the three classes of amines react differently with nitrous acid, HNO2 (prepared in situ from HCl + NaNO2)
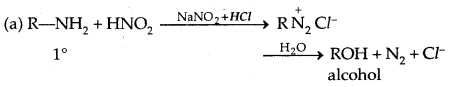
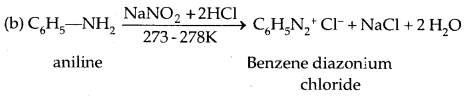
Secondary and tertiary amines react differently.
6. Reaction with aryl sulphonyl chloride: Benzensulphonyl chloride (C6H5SO2Cl) called Hinsberg’s reagent reacts with 1° and.2° amines.
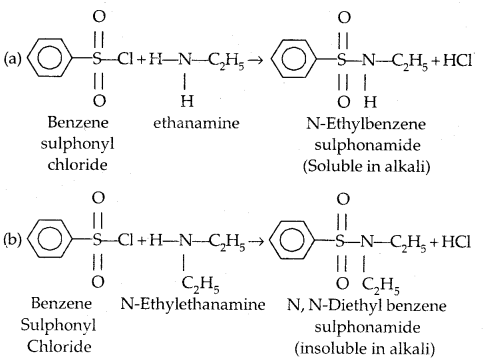
(c) Tertiary amines (R3N) do not react with benzene sulphonyl chloride. This property of 1°, 2° and 3° amines is made use of in their distinction and separation Now in place of benzene sulphonyl chloride,
p-toluenesulphonyl chloride is used.
![]()
7. Electrophilic Substitution: NH2 group fused in the ring is a powerful activating group and is ortho and para directing.
(a) Bromination:
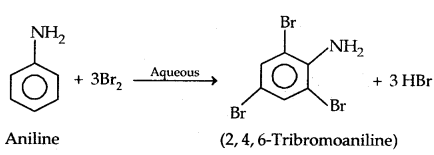
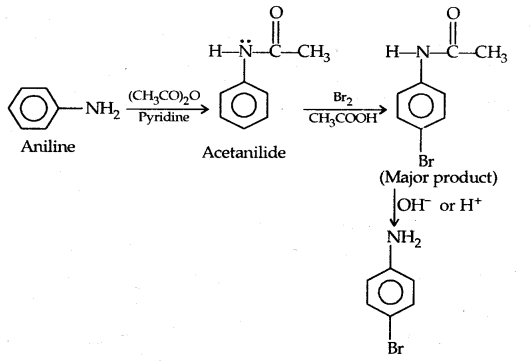
Protection of the highly activating -NH2 group can be done by acetylation with acetic anhydride in which case only monobromo substituted aniline is the product.
The lone pair of electrons on N of acetanilide interacts with oxygen atom due to resonance as shown below:
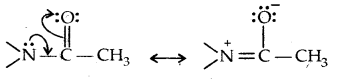
Hence the lone pair of electrons on nitrogen is less readily available for donation to the benzene ring by resonance. Thus the activating effect of – NHCOCH3 is less than that of the – NH2 group.
(b) Nitration: Direct nitration of aniline yields tarry oxidation products in addition to nitro derivatives.
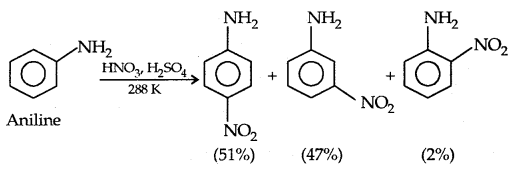
However, by protecting the amino group by acetylation reaction with acetic anhydride the nitration can be controlled and the para nitro derivative obtained as the major product.
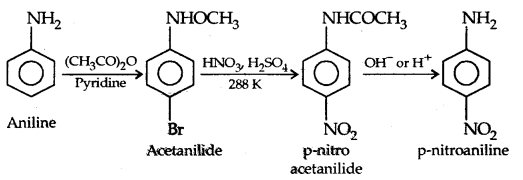
(c) Sulphonation:
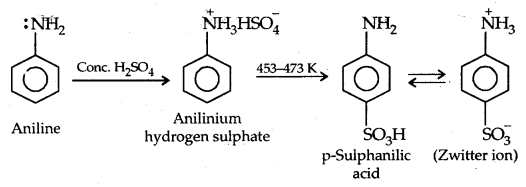
Aniline does not undergo Friedel Crafts reaction (alkylation and acetylation) due to the salt formation with AlCl3– the Lewis acid-which is used as a catalyst. The N of -NH2 acquires a positive charge and hence acts as a strong deactivating group for further reaction.
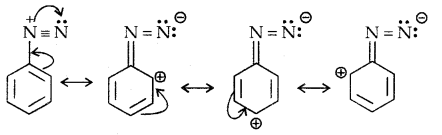
Diazonium Salts are of the type Ar N2+ X– where Ar stands for an aryl group and X– can be Cl–, Br, HSO4–, BF4– etc. N2+ group is called the diazonium group.
C6H5 N2+Cl– is called benzene diazonium chloride and C6H5 N2+ HSO4– is known as benzene diazonium hydrogen sulphate. The stability of the arene diazonium ion is explained on the basis of resonance.
Method of Preparation:
Physical Properties:
1. It is a colourless crystalline solid.
2. It is readily soluble in cold water and stable in it but reacts with water when warmed.
3. It decomposes easily in the dry state. Therefore it is used immediately after its preparation and is not stored.
Chemical Reactions A: Reactions involving displacement of Nitrogen -N2+ group is a very good leaving group. Therefore, it is substituted by other groups such as Cl–, Br–, I–, CN– and OH– which displace nitrogen from the aromatic ring.
1. Replacement by halide or cyanide ion: Sandmeyer Reaction
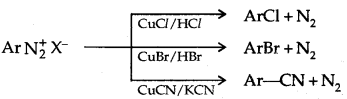
Alternatively, chlorine or bromine can also be introduced in the benzene ring by treating diazonium salt solution with corresponding halogen acid in the presence of copper powder. This is referred to as the Gatterman reaction.
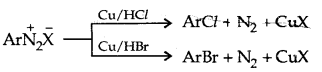
The yield in the Sandmeyer reaction is found to be better than the Gattermann reaction.
2. Replacement by Iodide Ion: Iodine is not easily introduced into the benzene ring directly, but, when the diazonium salt solution is treated with potassium iodide, iodobenzene is formed.
Ar N2+ Cl– + KI → Arl + KCl + N2
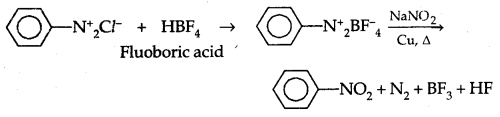
3. Replacement by Fluoride Ion: When arene diazonium chloride is treated with fluoroboric acid, arene diazonium fluoroborate is precipitated which on heating decomposes to yield aryl fluoride.
![]()
4. Replacement by H: Certain mild reducing agents like hypophosphorous acid (phosphinic acid) or ethanol reduce diazonium salts to arenes and themselves get oxidised to phosphorous acid and ethanol, respectively.
Ar N2+ Cl– + H3PO2 + H2O → ArH + N2 + H3PO3 + HCl
Ar N2+ Cl– + CH3CH2OH → ArH + N2 + CH3CHO + HCl
5. Replacement by hydroxyl group: If the temperature of the diazonium salt solution is allowed to rise up to 283 K, the salt gets hydrolysed to phenol.
Ar N2+ Cl– + H2O → ArOH + N2 + HCl
6. Replacement by – NO2 group: When diazonium fluoroborate is heated with aqueous sodium nitrite solution in the presence of copper, the diazonium group is replaced by the NO2 group.
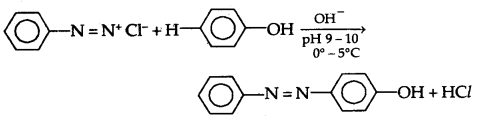
(B) Reactions involving retention of diazo group: Coupling Reactions
(a) Reaction with Phenol in the presence of a weakly alkaline medium results in the formation of p-hydroxy azobenzene which is an orange dye.
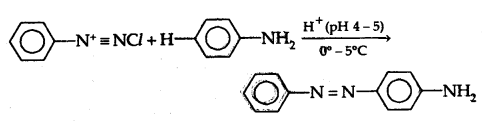
(b) Reaction with aniline: It reacts with aniline in a weakly acidic medium to form p-amino azobenzene (yellow dye)
→ Importance of Diazonium salts in the synthesis of Aromatic Compounds: These salts are very good intermediates used to introduce – F, – Cl, – Br,-I, – CN, – OH, – NOz groups into the benzene ring. Ar-F or Ar-I cannot be prepared by direct halogenation. The cyanobenzene which cannot be prepared by the SN reaction of chlorobenzene can be easily obtained from diazonium salts. These compounds are useful for preparing several azo dyes by coupling reactions.