By going through these CBSE Class 12 Chemistry Notes Chapter 15 Polymers, students can recall all the concepts quickly.
Polymers Notes Class 12 Chemistry Chapter 15
Polymers are macromolecules having high molecular mass [103 – 107 p]. They are formed by joining repeating structural units on a large scale. The repeating structural units are derived from some simple and reactive molecules known as monomers and are linked to each other by covalent bonds. The process of the formation of polymers from respective monomers is called polymerisation.
Classification of Polymers:
A. Based on the source.
- Natural Polymers: These are found in plants and animals. Examples are proteins, cellulose, starch, resins and rubber.
- Semi-synthetic Polymers: Cellulose acetate (rayon) and cellulose nitrate are examples of this category.
- Synthetic Polymers: Polyethene; nylon 6, 6; Buna-S are examples of man-made polymers.
B. Based on the structure of Polymers:
1. Linear Polymers: These polymers Consist of long and straight-chain repeating units derived from the monomers. The examples are high-density polyethene, polyvinyl chloride (PVC) etc. These are schematically represented as
2. Branched Chain Polymers: These polymers contain linear chains having some branches, e.g., low-density polyethene.
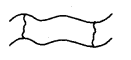
3. Cross-linked or Network Polymers: These are usually formed from bifunctional and trifunctional monomers, e.g., bakelite, melamine etc.
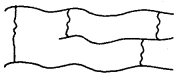
C. Classification Based on mode of Polymerisation:
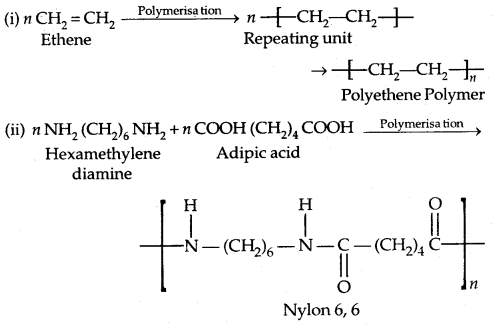
1. Addition Polymers: The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds, e..g, the formation of polyethene from ethene and polypropene from propene. In addition, polymers obtained from the same monomer are called Homopolymers, e.g., Polyethene.
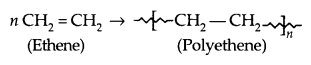
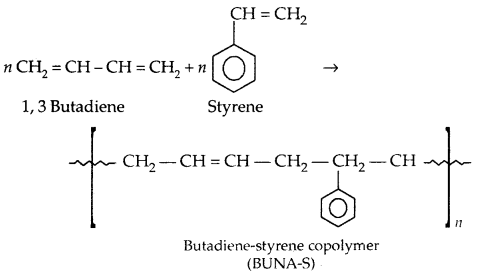
If two different units of monomers get added, they are called copolymers, e.g., Buna-S, Buna-N,
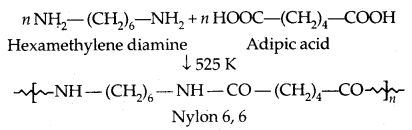
2. Condensation Polymers: The condensation polymers are formed by repeated condensation reaction between two monomeric units having different bifunctional and trifunctional groups with the elimination of small molecules like water, alcohol, hydrogen chloride etc. The formation of Nylon 6,6 is an example.
D. Classification based upon molecular forces:
1. Elastomers: These are rubber-like solids with elastic properties. The polymer chains are held together by weak intermolecular forces. They can be easily stretched. Examples are Buna-S, Buna-N, Neoprene etc.
2. Fibres: The intermolecular forces between the chains are strong hydrogen bonds. They have large tensile strength and are used to form thread forming crystalline solids. The examples are Nylon 6, 6 and polyesters.
3. Thermoplastic Polymers: In these polymers, the intermolecular forces are intermediate between those of elastomers and fibres. In these polymers, there is cross-linking between the chains. They soften on heating and harden on cooling. Common examples are polyethene, polystyrene polyvinyls etc.
4. Thermosetting Polymers: These polymers are cross-linked or heavily branched molecules, which on heating undergo expensive cross-linking in moulds and become infusible. They cannot be reused again. Common examples are bakelite and urea-formaldehyde resins etc.
E. Classification based on Growth Polymerisation: The addition and condensation polymers are nowadays also referred to as chain-growth polymers and step-growth polymers depending upon the type of polymerisation mechanism they undergo during their formation.
Types of Polymerization:
1. Addition Polymerization or Chain growth Polymerization: Here molecules of the same or different monomers add together on a large scale to form a polymer. It can proceed through the formation of free radicals or ionic species.
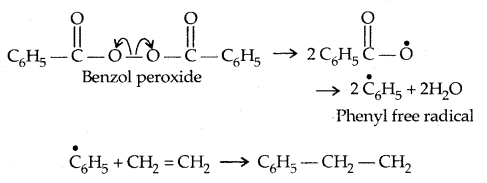
(a) Free Radical Mechanism: A variety of alkenes or dienes and their derivatives are polymerised in the presence of a free radical generating initiator (catalyst) like benzoyl chloride.
It consists up of the following three steps.
1. Chain-initiation Step:
2. Chain propagating step:
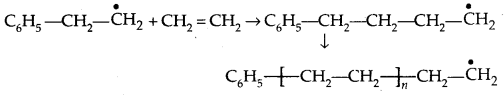
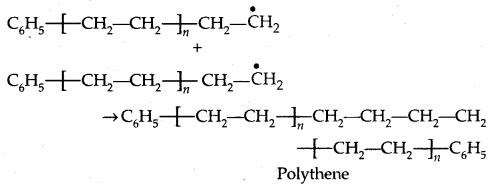
3. Chain terminating step:
(b) Preparation of some important Addition Polymers:
1. Polyethene: There are two types of polyethenes as given below:
1. Low-Density Polyethene (LDPE]:
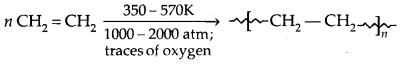
It is chemically inert and tough, but flexible and a poor conductor of electricity. It is used in the insulation of electric wires and the manufacture of squeeze bottles, toys and flexible pipes.
2. High-Density Polyethene (HDPE):
![]()
It has a high density. It is also chemically inert and tougher and harder. It is used for making buckets, dustbins, bottles and pipes.
2. Polytetrafluoroethene (Teflon):
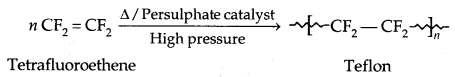
Chemically inert, it is resistant to attack by corrosive reagents. Used for making oil seals, gaskets and non-stick surface coated utensils.
3. Polyacrylonitrile:
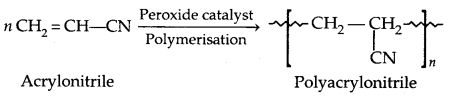
It is used as a substitute for wool in making fibres like Orlon or Acrilan.
→ Condensation Polymerization or Step-Growth polymerization: It involves a repetitive condensation reaction between two bifunctional monomers. It may result in the loss of simple molecules as H2O, alcohol etc.
1. Polyamides: Preparation of Nylons
1. Nylon 6,6:
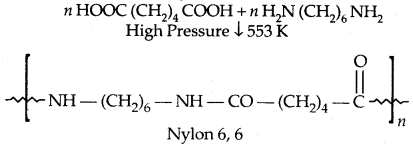
It is used in making sheets, bristles for brushes and in the textile industry.
2. Nylon 6: It is obtained by heating caprolactam with water at high temperature.
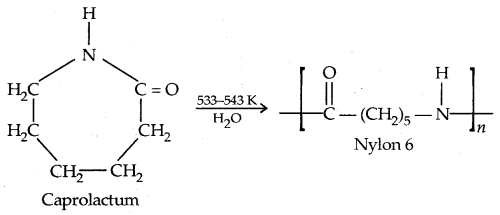
Nylon 6 is used for the manufacture of tyre cords, fabrics and ropes.
2. Polyesters: These are the polycondensation products of dicarboxylic acids and diols. The formation of terylene or dacron by the reaction between ethylene glycol and terephthalic acid is an example.
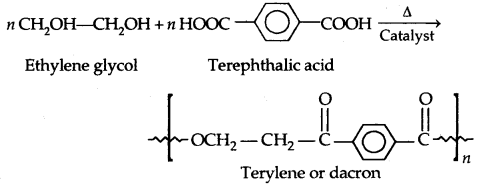
Dacron fibre (terylene) is crease-resistant and is used in blending with cotton and wool fibres and also as glass reinforcing materials in safety helmets etc.
3. Phenols formaldehyde polymer (Bakelite and related polymers): Phenol reacts with formaldehyde in the presence of dil. acid or base.
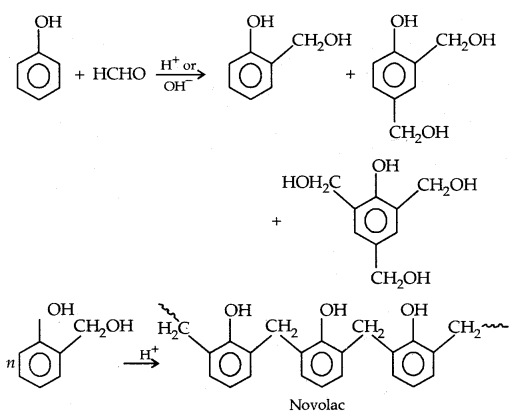
Novolac (used in paints) on heating with HCHO undergoes cross-linking to form an infusible solid mass called bakelite
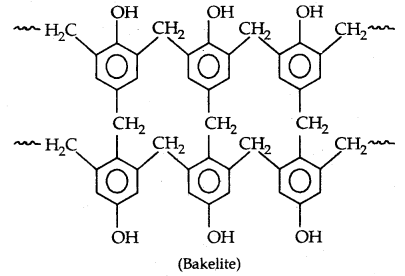
It is used for making combs, photograph records, electrical switches and handles of various utensils.
4. melamine-formaldehyde polymers: It is obtained by the condensation polymerisation of melamine and formaldehyde.
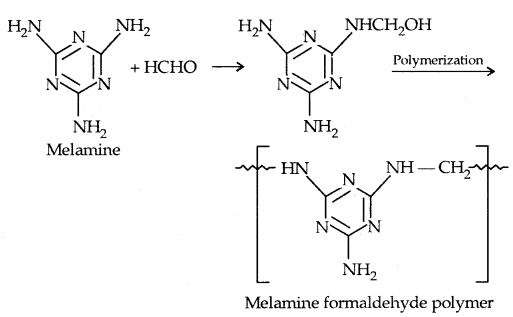
It is used in the manufacture of unbreakable cups and plates.
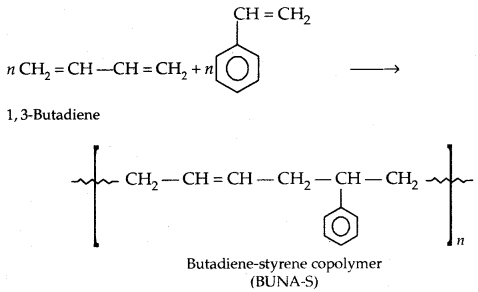
Copolymerization: A mixture of 1,3-butadiene and styrene form a copolymer: Butadiene-Styrene copolymer.
1. Natural rubber: It possesses elastic properties. It is a linear polymer of isoprene (2-methyl-l, 3-butadiene).
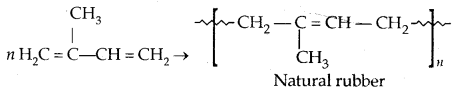
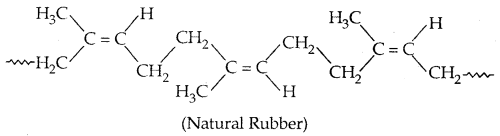
It is also called cis-1, 4-polyisoprene. It consists of various chains held together by weak van der Waals forces and has a coiled structure.
→ Vulcanisation of Rubber: To improve upon the physical properties of natural rubber, its vulcanisation is carried out. It consists of heating a mixture of raw rubber with sulphur and an appropriate additive at a temperature range between 373-415 K. On vulcanization sulphur forms cross-links at the reactive sites of double bonds and the rubber gets Stiffened. The probable structure of vulcanised rubber is:
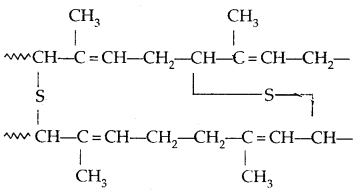
→ Synthetic Rubber: Synthetic rubbers are either homopolymers of 1, 3-butadiene derivatives or are copolymers of 1, 3-hutadíene or its derivatives with another unusual rated monomer.
1. Neoprene: It has superior qualities to natural rubber. It has better resistance to vegetable and mineral oils. It is used for the manufacture of conveyor belts, gaskets and hoses.
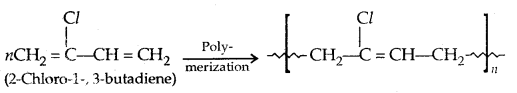
2. Buna-N: It is a copolymer of 1,3-butadiene and acrylonitrile in the presence of a peroxide catalyst.
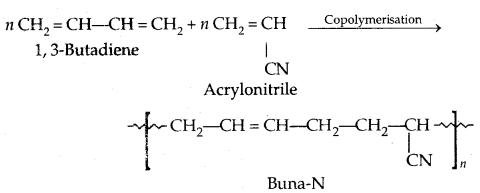
It is resistant to the action of petrol, lubricating oil and organic St .h ents. It is used is making oil seals tank living etc.
→ Molecular Mass of Polymers: Polymer properties are closely related to their molecular mass, size and structure. Its molecular mass is always expressed as an average.
It can be determined by chemical and physical methods.
- Weight-average molecular mass
- Number-average molecular mass.
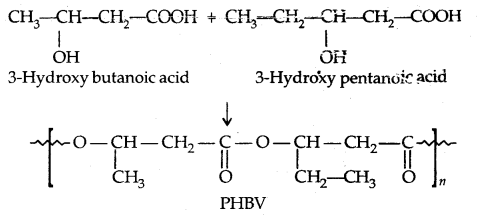
→ Biodegradable Polymers: A large number of polymers are non-biodegradable and are the reuse for environmental pollution. Nowadays, certain new biodegradable synthetic polymers have been designed and developed. Aliphatic polyesters are one of the important class of biodegradable polymers, e.g.,
→ Poly β-hydroxybutyrate-co-β-hydroxy valerate (PHBV): It is obtained by the copolymerisation of 3-hydroxybutyric acid and 3-hydroxy pentanoic acid.
PHBV undergosbateria1 degradation in the environment.
→ Nylon-2-Nylon 6: It is an alternating polyamide copolymer of glycine (H2N—CH2—COOH) and aminocaproic acid. (H2N (CH2)5 COOH) and is biodegradable.
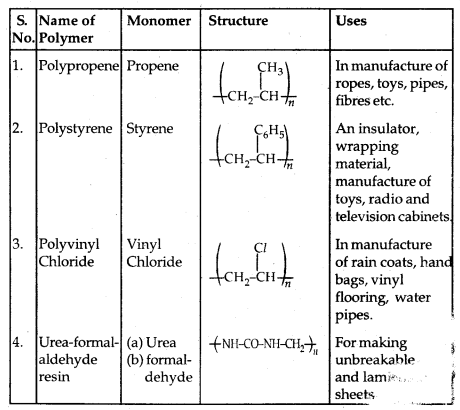
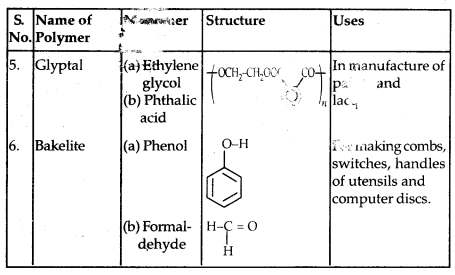
Some other commercially important Polymers along with their structures and uses are given below in the table: