By going through these CBSE Class 12 Chemistry Notes Chapter 16 Chemistry in Everyday Life, students can recall all the concepts quickly.
Chemistry in Everyday Life Notes Class 12 Chemistry Chapter 16
Medicines: Medical chemistry deals with the design and synthesis of drugs based on an undertaking of how these work in our body.
Drugs are chemicals of low molecular mass (~ 100-500 μ). They interact with macromolecular targets and produce a biological response. When the biological response is effective and useful, these chemicals are called medicines and are used in the treatment, diagnosis, and prevention of diseases. In larger doses than recommended, they are potential poisons. The use of chemicals for therapeutic effect is called Chemotherapy.
Designing of a Drug: Two considerations arise
- Drug target,
- drug metabolism.
1. Drug target: The biological macromolecules such as carbohydrates lipids, proteins, nucleic acids with which drugs interact are called targets. The correct choice of the molecular target for a drug is important to obtain a desired therapeutic effect.
2. Drug metabolism: A drug travels through the body in order to reach the target. So its design should be such that it reaches the target without being metabolized in between. Also, after its action, it should be excreted without causing harm to the body.
Compounds from which drugs are designed are called lead compounds. These lead compounds may be obtained from natural sources such as plants, trees, bushes, venoms, and metabolites of microorganisms or they may be synthesized in order to improve drug activity and to have minimum side effects, mechanisms of drug action in the biological systems are also considered while drug designing.
Classification of Drugs:
1. On the basis of Pharmacological effect: It is useful for doctors. For example, analgesics have a pain-killing effect, antiseptics kill or arrest the growth of microorganisms.
2. On the basis of action on a particular biochemical process: All antihistamines inhibit the action of the compound histamine, which causes inflammation in the body.
3. On the basis of chemical structure: Drugs classified in this way share common structural features and often have similar pharmacological activity. For example, sulphonamides have common structural features given below and are mostly antibacterial.
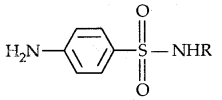
(Sfructuralfratures of Suiphona mides)
4. On the basis of molecular targets: This classification is most useful for medicinal chemists. Various enzymes and receptors in the cell are some of the common drug targets.
Interaction of drugs with targets: Proteins that perform the role of biological catalysts in the body are called enzymes. Proteins that are important to a communication system in the body are called receptors. Tires enzymes and receptors serve as drug targets among others.
Enzymes as Drug Targets:
(a) Catalytical activity of enzymes: Enzymes perform two major functions:
1. The first function of an enzyme is to hold the substrate for a chemical reaction. Active sites of enzymes hold the substrate molecule in a suitable position so that it can be attacked by the reagent effectively.
Substrates bind to the amino acid residues of the protein present on the active site of the enzyme through a variety of interactions such as ionic bonding, hydrogen bonding, van der Waals interaction of dipole-dipole interaction (Fig.).
These binding interactions should be strong enough to hold the substrate long enough so that the enzyme can catalyze the reaction, but weak enough to allow the products to depart after their formation.
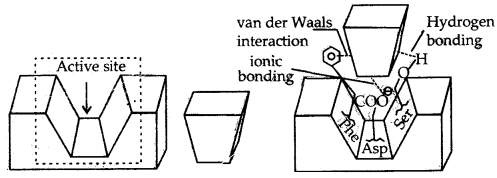
(a) Active site of an enzyme,
(b) substrate
(c) Substrate held in the active site of the enzyme
(a) The second function of the enzyme is to provide functional groups that will attack the substrate and carry out a chemical reaction. This function is carried out by some other amino acid residues of protein present on the active site of the enzyme.
These provide free functional groups to attack the substrate and bring about chemical reactions. For example, if amino acid, serine is present nearby the substrate held on the active site, then its – OH group is free to act as a nucleophile in the enzyme-catalyzed reaction.
(b) Interaction of drugs with enzymes: Drugs inhibit the activity of the enzymes and so are called Enzyme Inhibitors. Enzyme inhibitors can block the binding site and prevent the binding of substrate or these can inhibit the catalytical activity of the enzyme.
(c) Prevention of attachment of natural substrate in the active site by drugs: Drugs inhibit the attachment of substrate on the active site of enzymes in two different ways explained below:
Drugs compete with the natural substrate for the active sites. Such drugs are called competitive inhibitors.
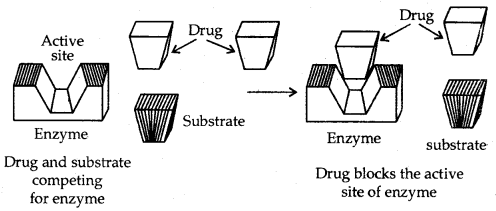
(Drug and substrate competing for the active site)
2. On the other hand, some drugs do not bind to the active site. These bind to a different site of enzyme which is called the allosteric site. This binding of inhibitors at the allosteric sites changes the shape of the active site in such a way that the substrate cannot recognize it.
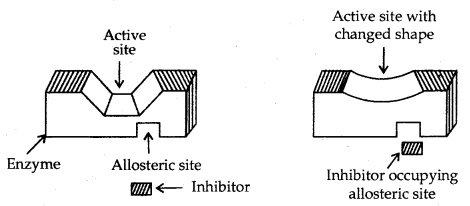
[Noncompetitive inhibitor changes the active site of the enzyme after binding at the allosteric site]
If the bond formed between enzyme and inhibitor is a strong covalent bond and cannot be broken easily then the enzyme is blocked permanently. The body then degrades the enzyme inhibitors complex and synthesizes new enzymes.
Receptors as Drug Targets:
→ Location of receptor in the animal cell: Receptors are proteins that are crucial to the body’s communication process. The majority of these are embedded in cell membranes.
Receptor proteins are embedded in the cell membrane in such a way that their small part possessing active site projects out of the surface of the membrane and opens on the outer region of the cell membrane.
→ Transfer of message into the cell by receptors: Neurotransmitters communicate messages in the body between the 3 neurons and that between neurons to muscles. These chemical messengers are received at the binding site of the receptor protein. To accommodate messenger, the shape of the receptor changes. This brings about the transfer of the message into the cell. Thus, chemical messenger gives a message to the cell without entering the cell.
Two types of chemical messengers are involved in the message transfer:
- Hormones
- neurotransmitters
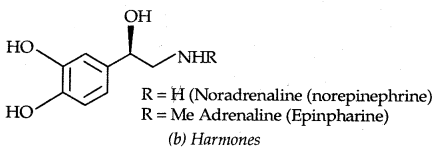
1. Hormones: Adrenaline (epinephrine) is an example of hormone. It is released from the adrenal medulla in situations of stress or danger.
2. Neurotransmitters are small molecules such as acetylcholine, dopamine, and serotonin.
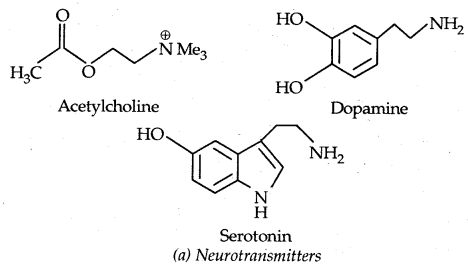
→ Interaction of Drugs: Receptors that interact with one specific chemical messenger may differ slightly in their binding sites.
For example, there are two types of adrenergic receptors named a-adrenergic receptors and β-adrenergic receptors. These differ slightly in the structure of their binding sites, but both of these receptors can bind epinephrine.
Drugs that bind to the receptor site and inhibit its natural function are called antagonists. There are other types of drugs that mimic the natural messenger by switching on the receptor. They are called agonists.
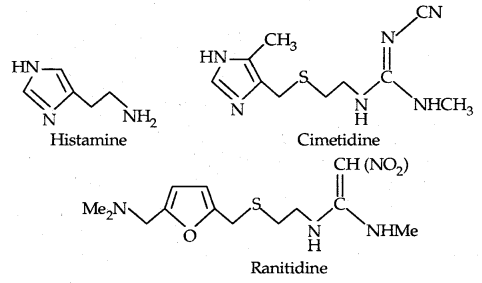
→ Side-effects caused by drugs: Side effects are caused when a drug binds to more than one type of receptor, e.g., the serotonin receptor is a target for some anti-depressant drugs. Side effects can arise if the drug interacts with histamine or acetylcholine.
Types erf Drugs:
1. Antacids: If acid is produced in excess in the stomach, it causes irritation and pain and in severe cases, ulcers are produced. Histamine stimulates the secretion of pepsin and hydrochloric acid. A drug like cimetidine (Tagamet) and ranitidine (Zantac) was designed to prevent the interaction of histamine with the receptors present in the stomach wall. This resulted in the release of a lesser amount of acid.
2. Antihistammines: Histamine is a potent vasodilator. It has various functions. It contracts the smooth muscles in the bronchi and gut and relaxes other muscles. It is also responsible for the nasal congestion associated with common colds and allergic response to pollen. Synthetic drugs brompheniramine (Dimetapp) and terfenadine (Seldane) act as antihistamines.
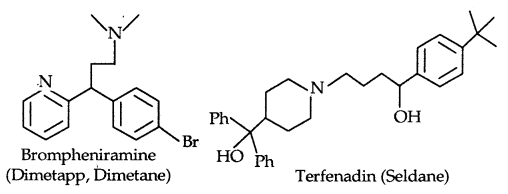
The above-mentioned antihistamines do not affect the secretion of acid in the stomach. It is because that antiallergic and antacid drugs work on different receptors.
3. Neurologically Active Drugs: Tranquilizers and analgesics are neurologically active drugs.
These affect the message transfer mechanism from the nerve to the receptor.
(a) Tranquilizers are a class of compounds used for the treatment of stress, mild and severe mental diseases. They relieve stress, anxiety irritability, and excitement by inducing a sense of well-being.
→ They act on the central nervous system (CNS): Noradrenaline is one of the neurotransmitters that plays role in mood changes. If its level is low for some reason, the signal sending activity becomes low and the person suffers from depression.
Antidepressant drugs, in such cases, inhibit the enzymes which catalyze the degradation of noradrenaline. If the enzyme is inhibited, this important neurotransmitter is slowly metabolized and can activate its receptor for longer periods of time, thus countering the effect of depression. Iproniazid and phenelzine are two such drugs.
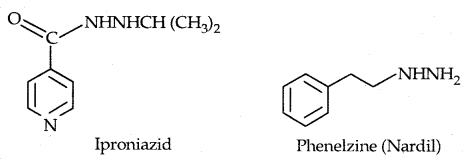
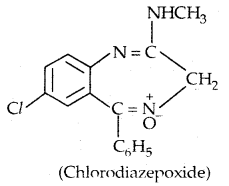
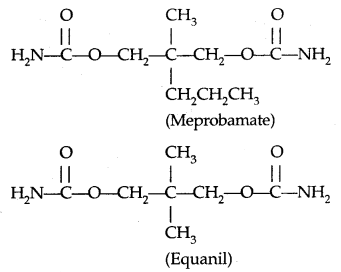
Some tranquilizers namely, Chlorodiazepoxide and Meprobamate are relatively mild tranquilizers suitable for relieving tension. Equanil is used in controlling depression and hypertension.
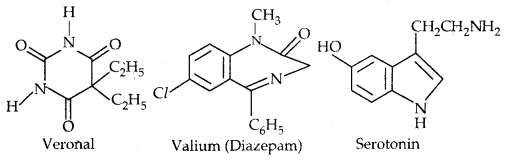
→ Barbiturates: The derivatives of barbituric acid are hypnotic- sleep-producing agents. Some of them are Veronal, Valium and Serotonium.
(b) Analgesics: are the drugs that reduce or abolish pain without causing impairment of consciousness, mental confusion, or some other disturbance of the nervous system.
They are of two types:
1. Non-narcotic (non-addictive) drugs: Aspirin and paracetamol belong to the class of non-addictive analgesics. These drugs have many other effects such as reducing fever (antipyretic) and preventing platelet coagulation. Aspirin is helpful to prevent heart attacks,
2. Narcotic analgesics: like morphine, heroin, codeine relieve pain and produce sleep in medicinal doses, and in excess are fatal. These analgesics are chiefly used for the relief of post-operative pain, cardiac pain, and pains of terminal cancer and in childbirth.
4. Antimicrobials: Disease may be caused by bacteria, viruses, etc. P. Ehrlich who developed the medicine Salvarsan for the treatment of syphilis found that the -As = As – linkage present in arsphenamine (salvarsan) resembles the -N = N- linkage present in azo-dyes in the sense that N atom is present in place of As. He was successful in 1932 in preparing the first effective antibacterial agent Prontosil which resembles the structure of the compound salvarsan.
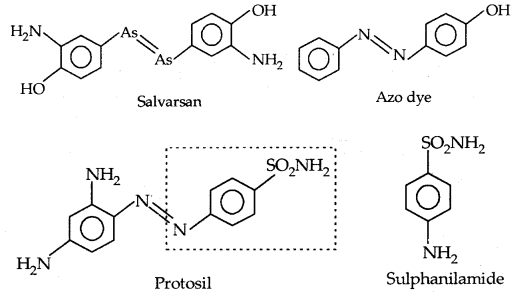
[The structures of salvarsan and prontosil and azo dye showing structural similarity]
This led to the study of the relation between structure and activity. It was found that part of the proposal molecule (shown in the box) in the form of p-amino benzene sulphonamide (Sulphanilamide) has antibacterial activity. The led to the discovery of Sulpha drugs.
Antimicrobials control microbial diseases in three ways:
(a) a drug that kills the organism in the body (bactericidal).
(b) a drug that inhibits or arrests the growth of organisms (bacteriostatic) and
(c) increasing immunity and resistance to infection in the body,
5. Antibiotics: It is a substance (produced wholly or partly by chemical synthesis) that in low concentration inhibits the growth or destroys microorganisms by intervening in their metabolic processes.
The first antibiotic discovered by Alexander Fleming’s Penicillin from the mold Penicillium Notatum.
The antibiotics can be either bactericidal or bacteriostatic.
| Bactericidal | Bacteriostatic |
| Penicillin | Erythromycin |
| Aminoglycosides | Tetracycline |
| Ofloxacin | Chloramphenicol |
Broad Spectrum antibiotics are medicines effective against several types of harmful microorganisms, e.g., tetracycline, chloramphenicol.
6. Antiseptics and disinfectants: Antiseptics and disinfectants are also the chemicals which either kill or prevent the growth of microorganism.
Antiseptics are applied to living tissues such as wounds, cuts, ulcers, and diseased skin surfaces. Examples are Furacine, Soframicine, etc. Dettol is a mixture of Chloroxylenol and terpineol. Bithinol is added to soaps to impart antiseptic properties. Iodine is a powerful antiseptic. Its 2-3% solution in alcohol-water solution is known as tincture of iodine. It is applied to wounds. Iodoform is also used as an antiseptic for wounds. Boric acid (H3P03) in dilute solution (aqueous) is a weak antiseptic for the eyes.
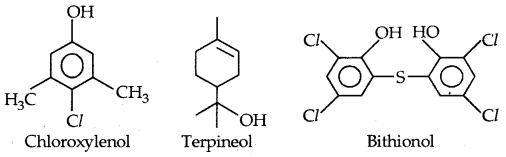
Disinfectants are applied to inanimate objects such as floors, drainage systems, instruments, etc. The same substance can act as an antiseptic as well as a disinfectant by varying the concentration. For example, 0.2 percent situation of phenol is an antiseptic while it’s one percent solution is disinfectant.
Chlorine in the concentration of 0.2 to 0.4 ppm and S02 in very low concentration are disinfectants.
7. Antifertility Drugs: Norethindrone is an example of synthetic progesterone (a type of hormone) derivative most widely used as an antifertility drug for birth control. The estrogen derivative is used in combination with progesterone derivative is ethynylestradiol (Novestrol).
→ Chemicals in Food: To enhance the shelf life of food to make it more appealing and sometimes more nutritive, chemicals are added to it.
They are:
- Food colors,
- Flavors and sweeteners,
- Fat emulsifiers and stabilizing agents,
- Flour improvers antistaling agents and bleaches,
- Antioxidants,
- Preservatives,
- Nutritional supplements such as minerals, vitamins, and amino acids.
Except for category (7), none of the chemical additives have any nutritive value.
→ Artificial Sweetening agents: Ortho-Sulphobenzimide (saccharine)
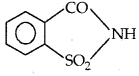
is an artificial sweetener and mass/mass, it is 550 times as sweet as cane sugar. It is excreted from the body in the urine unchanged and appears to be entirely harmless and inert and so is of great value to diabetic persons and people who need to control the intake of calories.
Other artificial sweeteners are aspartame (100 times sweet as sugar), sucralose (600 times) alitame (2000 times as sweet as sugar).
→ Preservatives: In addition to class I preservatives like salts, sugar, and vegetable oils, the most common class II preservative is sodium benzoate
![]()
which is used in limited quantities and is metabolized in the body.
→ Chemistry of Cleansing Agents:
1. Soaps: Soaps are sodium or potassium salts of long-chain fatty acids, e.g., stearic acid, oleic acid, and palmitic acid. Soaps are obtained by the saponification of triglycerides of fatty acids.
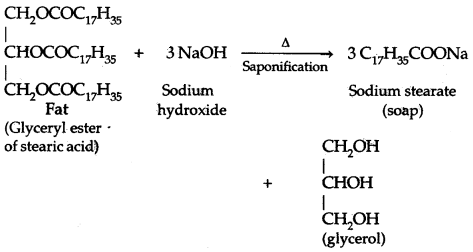
Potassium soaps are softer than sodium soaps.
Types of Soaps: Toilet Soaps are prepared by using better grades of fats and oils and excess alkali is removed. Colour and perfumes are added. Transparent Soap is made by dissolving the soap in ethanol and then evaporating the excess solvent.
In medicated soaps, substances of medicinal value are added. ! Shaving soaps contain glycerol to prevent rapid drying. Laundry soaps t contain fillers like sodium proximate, sodium silicate borax, and sodium \ carbonate.
Soaps do not work in hard water as soaps react with Ca2+ and Mg2+ ions present in hard water to produce curdy precipitate or scum,
![]()
2. Soapless detergents: Soapless detergents are cleansing agents; which have all the properties of soaps, but they actually do not contain; soap. They are useful in hard water also.
Synthetic detergents are mainly of three types:
- Anionic detergents
- Cationic detergents
- Non-ionic detergents
1. Anionic Detergents are sodium salts of sulfonated long-chain alcohols.
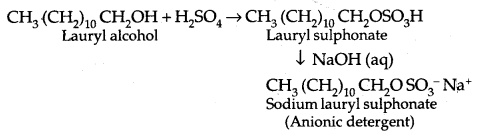
In anionic detergents, the anionic part of the molecule is involved in the cleansing action.
2. Cationic Detergents: Cationic detergents are acetates, chlorides, or bromides of quaternary ammonium salts. An example is cetyltrimethylammonium bromide:
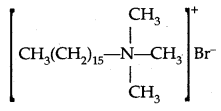
Cationic detergents are expensive and due to their germicidal properties, they are used as hair conditioners.
3. Non-ionic Detergents: Stearic acid reacts with polyethylene glycol to form non-ionic detergents.
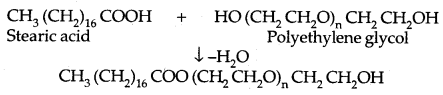
Liquid dishwashing detergents are non-ionic types. Detergents containing highly branched hydrocarbon chains are not easily biodegradable.