By going through these CBSE Class 12 Chemistry Notes Chapter 7 The p-Block Elements, students can recall all the concepts quickly.
The p-Block Elements Notes Class 12 Chemistry Chapter 7
The p-block elements are placed in groups 13 to 18 of the periodic table. Their valence shell electronic configuration is ns2np6 to ns2np6 (except He which has 1s2 electronic configuration.)
Group 15 Elements: Group 15 elements include Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb) and Bismuth (Bi).
Table 7.1: Atomic and Physical Properties of Group 15 Elements
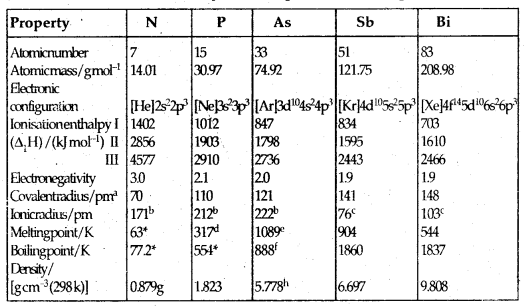
αE111 single bond (E = element); bE3-; cE3+; dWhite phosphorus; eGrey α-format 38.6 atm of Sublimation temperature; g At 63 K h Grey α-form; Molecular N2
As we go down the group, there is a shift from non-metallic to the metallic character through metalloids character. N and P are non-metals, AS and Sb are metalloids and Bi is a typical metal.
→ Occurrence: N2 comprises 78% by volume of the atmosphere. In the earth’s crust, it occurs as sodium nitrate (NaNO3) called Chile saltpetre and potassium nitrate (KNO3) called Indian saltpetre. It is found in the form of proteins in plants and animals. Phosphorus (P) occurs in minerals of the apatite family Ca9 (PO4)6. CaX2 [X = F, Cl, or OH] e.g. fluorapatite Ca9 (PO4)6. CaF2 which are the main components of the phosphate rocks. P is an important constituent of animal and plant matter and is present in bones and living cells.
→ Electronic Configuration: The valence shell electronic configuration of these elements is ns2np3. The s orbital is completely filled and p orbitals are exactly half-filled (px1 py1 Pz1). Thus their electronic configuration is extra stable.
→ Atomic and Ionic Radii: Covalent and ionic radii increase in size down the group due to the addition of the orbit each time. There is a considerable increase from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and /or f orbitals in heavier members.
→ Ionization Enthalpy: I.E. values of Group 15 elements is much greater than that of Group 14 elements due to the extra-stability of half-filled p-orbitals and smaller size. It decreases from top to bottom within the group due to a gradual increase in atomic size. The order of successive ionisation enthalpies is ΔiH1 < ΔiH2 < ΔiH3.
→ Electronegativity: Electronegativity decrease down the group. The difference is not much pronounced amongst the heavier elements.
→ Physical Properties: All the elements are polyatomic. Dinitrogen (N2) is a diatomic gas while others like P4 etc. are solids. The b. pts; in general, increase from top to bottom in the group, but them. pts increase up to As and then decrease up to Bi.
Except for Nitrogen, all the elements show allotropy.
Chemical Properties:
Oxidation states and trends in chemical reactivity: Tire common oxidation states of these elements are – 3, + 3 and + 5. The tendency to exhibit – 3 oxidation state decreases down the group due to an increase in size and metallic character.
N does not show + 5 oxidation state due to the absence of d-orbitals and Bi does not show + 5 oxidation state due to the Inert pair Effect. The only well characterised Bi (V) compound is BiF5. N exhibits + 1, + 2, + 4 oxidation states also when it reacts with oxygen. P also exhibits + 1 and + 4 oxidation states in some oxoacids.
In the case of N, all oxidation states from + 1 to + 4 tend to disproportionate into acid solution1. For example
3 HNO2 → HNO3 + 2 NO + H2O
Similarly, in the case of P nearly all intermediate oxidation states disproportionate into + 5 and – 3 both in alkali and acid,
Anomalous properties of Nitrogen: N differs from its congeners due to
- its smaller size.
- high electronegativity and high ionisation enthalpy.
- non-availability of d-orbitals.
Nitrogen has a unique ability to form an-pit multiple bonds with itself and with other elements having small size and having high electronegativity (e.g., C, O)
N2 has a triple bond: N ≡ N [one a and two K bonds]
∴ Its bond enthalpy (941.4 kJ mol-1) is very high.
P and As can form dπ-pπ bonds with transition metals.
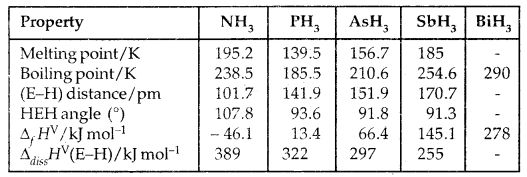
1. Reactivity towards hydrogen: All the elements of Group 15 form hydrides of the type EH3 where E = N, P, As, Sb, Bi. The stability of hydrides decreases from NH3 to BiH3 and hence reducing character increases from NH3 to BiH3. Basicity of hydrides also decreases in the order NH3 > PH3 > AsH3 > SbH3 > BiH3.
Table 7.2: Properties of Hydrides of Group 15 Elements
2. Reactivity towards oxygen: All these elements form oxides: E2O3 and E2O5. The oxide in the higher oxidation state is more acidic.
Their acidic character decreases down in a group. The oxides of the type EO3 of N and Pare purely acidic, that of As, Sb amphoteric and those of Bi predominantly basic.
3. Reactivity towards halogens:
2E + 3X2 → EX3
2E + 5X2 → 2EX5
N does not form pentahalide due to absence of d-orbitais. All trihalides are stable except that of N.
Pentahalides are more covalent than trihalides.
4. Reactivity towards metals: These elements combine with metals showing – 3 oxidation state in compounds like Ca3N2, Ca3P2, Mg3Bi2.
Dinitrogen, N2:
Preparation:
1. Commercially dinitrogen is produced by the liquefaction and fractional distillation of air when N2 distils the first.
2. Lab. Method:
NH4Cl (aq) + NaNO2 (aq) → N2 (g) + 2H2O (l) + NaCl (aq)

3. Pure N2 can be obtained from sodium or barium azide.
![]()
→ Properties: Dinitrogen is a colourless, odourless, tasteless and non-toxic gas. It has two stable isotopes: 14N, 15N. It has very low solubility in water.
Because of the high bond enthalpy of N ≡ N, dinitrogen is rather inert at room temperature.
At high temperature, it directly combines with some metals to form largely ionic nitrides and with non-metals, covalent nitrides.
Haber’s process:
Uses:
- Manufacture of NH3 and Calcium Cyanamide.
- To create an inert atmosphere.
- Liquid N2 is used as a refrigerant,
Ammonia:
Preparation:
1. From Urea:
NH2CONH2 + 2H2O → (NH4)2 CO3 → 2NH3 + H2O + CO2
2. From ammonium salts and caustic soda/lime.
2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O + CaCl2
(NH4)2 SO4 + 2 NaOH → 2NH3 + 2H2O + Na2SO4
3. On a large scale, it is prepared by Haber’s process.
![]()
The pressure is 200 × 105 Pa and iron oxide with small amounts of K2O and Al2O3 is used as a catalyst,
Properties of Ammonia:
- It is a colourless gas with a pungent smell.
- It shows hydrogen bonding in solid and liquid states.
Structure of NH3:
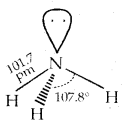
3. It is highly soluble in water. Its aqueous solution is weakly basic.
NH3 (g) + H2O (l) ⇌ NH4+ (aq) + OH– (aq)
4. It forms ammonium salts with acids.
NH3 + HCl → NH4Cl
5. As a weak base it precipitates the hydroxides of many metals
from their salt solutions, e.g.,
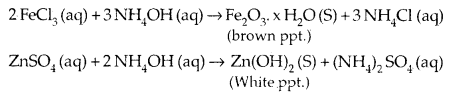
6. Due to its tendency to donate a pair of electrons on N, it acts as a Lewis-Base, forms complexes with Cu2+, Ag+.
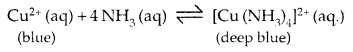
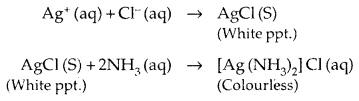
Uses:
- Production of nitrogenous fertilizers.
- Production of nitric acid.
- Liquid ammonia is a non-aqueous solvent.
- Liquid NH3 is a referigerant.
Oxides of Nitrogen: Nitrogen forms a number of oxides in different oxidation state. The details of names, formulae, ox. state, preparation, colour etc. are given:
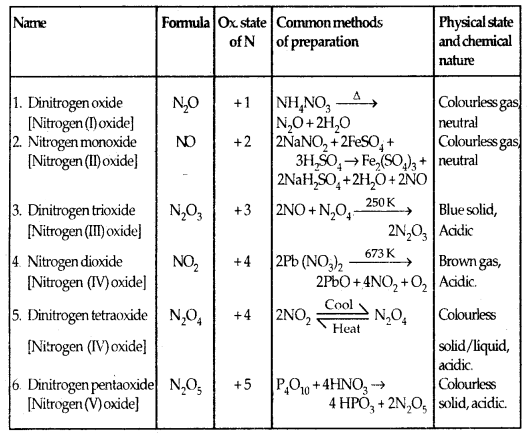
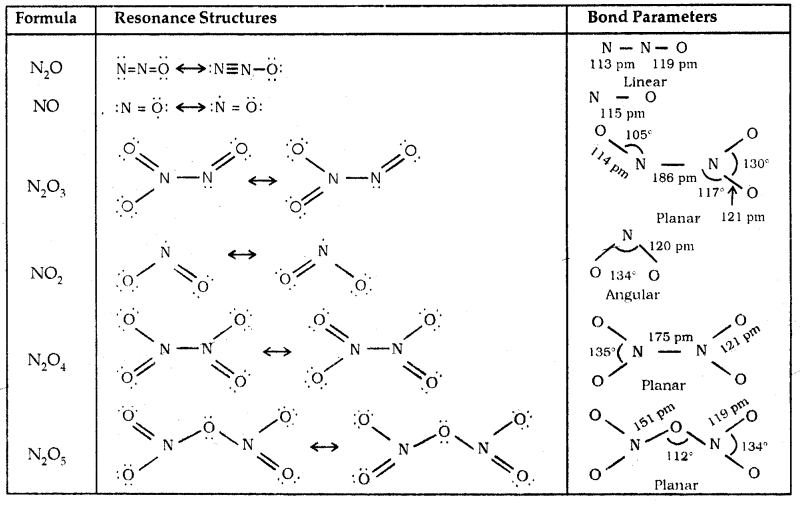
Lewis dot main resonance structures and bond parameters of oxides are given on the next page.
Table 7.4 Structures of Oxides of Nitrogen
Nitric Acid, HNO3
Preparation: It is prepared in the laboratory by heating NaNO3 or KNO3 and cone. H2SO4.
![]()
Method of manufacture of Nitric acid by Ostwald’s process.
Step:
1. Catalytical oxidation of NH3 by O, or air.
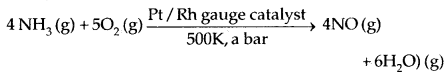
2. NO thus formed combines with 02 giving NO2
2NO (g) + O2 (g) ⇌ 2NO2(g).
3. N02 SO formed dissolves in water to give HNO3.
3NO2 + H2O(l) → 2 HNO3 + NO (g)
Structure of HNO3: In the gaseous state, HNO3 exists as a planar molecule.
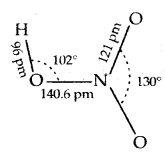
In an aqueous solution, it behaves as a strong acid.
HNO3 (aq) + H2O (Z) → (aq) + NO3 (aq).
Concentrated HNO3 is a strong oxidizing agent.
→ Reaction with Cu:
3Cu + 8 HNO3 (dil) → 3 Cu (NO3)2 + 2 NO + 4 H2O
Cu + 4 HNO3 (cone.) → Cu (NO3)2 + 2NO2 + 2H2O
→ Reaction With Zinc
4 Zn + 10 HNO3 (dil.) → 4 Zn (NO3)2 + 5 H2O + N2O
Zn + 4HNO3 (cone.) → Zn (NO3)2 + 2H2O + 2NO2
→ Reaction with I2
![]()
→ Reaction with Carbon
C + 4 HNO3 → CO2 + 2H2O + 4NO2
→ Reaction with Sulphur
S8 + 48 HNO3 (Cone.) → 8H2SO4 + 48 NO2 + 12 H2O
→ Reaction with Phosphorus
P4 + 20 HNO3 → 4H3PO4 + 20 NO2 + 4H2O
→ Brown Ring Test for nitrates (NO3–)
NO3– + 3 Fe2+ + 4 H+ → NO + 3 Fe3+ + 2H2O.
![]()
Uses of Nitric acid:
- Oxidant in the laboratory.
- manufacture of ammonium nitrate for fertilizers.
- manufacture of explosive like TNT, nitroglycerine. Phosphorus: Phosphorus exists in three important allotropic forms: white, red and black.
White Phosphorus: White Phosphorus consists of discrete tetrahedral molecules as shown:
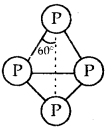
White phosphorus P4
Red Phosphorus: Red Phosphorus is obtained by heating white phosphorus at 573 K in an inert atmosphere for several days. It is much less reactive than white phosphorus. It is polymeric, consisting of P4 tetrahedral linked together as shown in Fig. below.
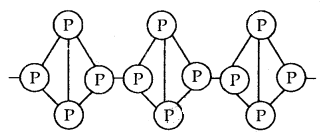
Red Phosphorus
Black Phosphorus has two forms α-black phosphorus and β-black phosphorus. α-Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803 K. It can be sublimed in air and has opaque monoclinic or rhombohedral crystals. It does not oxidise in the air. β-Black phosphorus is prepared by heating white phosphorus at 473 K under high pressure. It does not bum in the air up to 673 K.
Phosphine (PH3) Preparation:
1. From calcium phosphide and H2O/dil. HCl
Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3.
Ca3P2 + 6HCl → 3CaCl2 + 2PH3.
2. Lab. Method: By heating white phosphorus with cone. NaOH in an inert atom of CO2.
P4 + 3NaOH +- 3H2O → PH3 + NaH2PO2 (Sod. hypophosphite)
Properties:
- Colourless gas with rotten fish smell, highly poisonous.
- Explodes in contact with traces of oxidizing agents like HNO3 Cl2 etc.,
- Slightly soluble in water.
- When absorbed in CuSO4 or HgCl2 solutions, phosphides are obtained.
3 CuSO4 + 2 PH3 → Cu3P2 + 3H2SO4
3HgCl2 + 2PH2 → Hg2 P2 + 6HCl. - PH3 is weakly basic like NH3.
PH3 + HBr → PH4Br.
Phosphorus halides: P forms two types of halides, PX3 [X = F, Cl, Br, I]and PX5[X = F,Cl,Br]
Phosphorus trichloride (PCl3)
Preparation:
P4 + 6Cl2 → 4PCl3.
Properties:
- Colourless oily liquid.
- Hydrolysis in the presence of mixture.
PCl3 + 3H2O → H3PO3 + 3HCl
Shape: It has a pyramidal shape as shown in which phosphorus is sp3 hybridized.
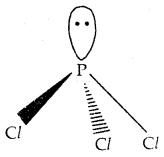
Shape of PCl3
Phosphorus pentachloride (PCl5)
Preparation:
![]()
Properties:
- It is a yellowish-white powder.
- In moist air, it hydrolyses first to PoCl3, and then H3PO4.
PCl5 + H2O → POCl3 + 2 HCl
POCl3 + 3H2O → 4 H3PO4 + 3HCl - On heating, it sublimes but decomposes on stronger heating.
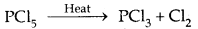
In gaseous and liquid phases, it has a trigonal bipyramidal structure shown below. The three equatorial P-Cl bonds are equivalent, while the two axial bonds are longer than equatorial bonds. This is due to the fact that the axial bond pairs suffer more repulsion as compared to equatorial bond pairs.
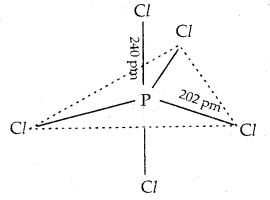
In the solid-state, it exits as an ionic solid, [PCl4]+[PCl6]– in which the cation, [PCl4]+ is tetrahedral and the anion, [PCl6]+ octahedral.
Oxo-Acids of Phosphorus: P forms a number of oxo-acids like
- Hypophosphorous acid (H3PO2) (ox. state + 1)
- Orthophosphoric acid (H3PO3) (ox. state + 3)
- Pyrophosphrus acid (H4P2O5) (ox. state + 3)
- Hypophosphoric acid (H4P2O6) (ox. state +4)
- Orthophosph’oric acid (H3PO4) (ox. state + 5)
- Pyrophosphoric acid (H4P2O7) (ox. state +5)
- Metaphosphoric acid (HPO3) (ox. state + 5)
The compositions of the oxo-acids are interrelated in terms of loss or gain of H2O molecule or oxygen atom.
These acids in the + 3 oxidation state of P tend to disproportionate as follows:
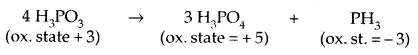
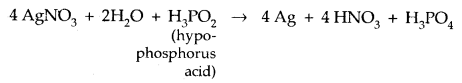
The acids which contain the P-H bond are strong reducing agents.
Group 16 Elements: Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te) and Polonium (Po) constitute Group 16 of the periodic table. They are also called Chalcogens (ore-forming).
→ Occurrence: Oxygen is the most abundant of all the elements of the earth. Dry air contains 20.95% oxygen by volume. However Sulphur is present in the earth’s crust to 0.03 – 0.1% only. Combined Sulphur is present in gypsum CaSO4. 2H2O, Epsom salt MgSO4.7H2O. Selenium and tellurium are also found as metal selenides and tellurides in sulphide ores.
→ Electronic Configuration: The elements of Group 16 have six electrons in the outermost shell and have ns2np4 general electronic configuration.
→ Atomic and Ionic Radii: Due to the increase in the no. of shells, atomic and ionic radii increase from top to bottom, The size of the oxygen atom is, however, exceptionally small.
→ Ionisation Enthalpy: Ionisation enthalpy decreases down the group.
Table: Some Physical Properties of Group 16 Elements
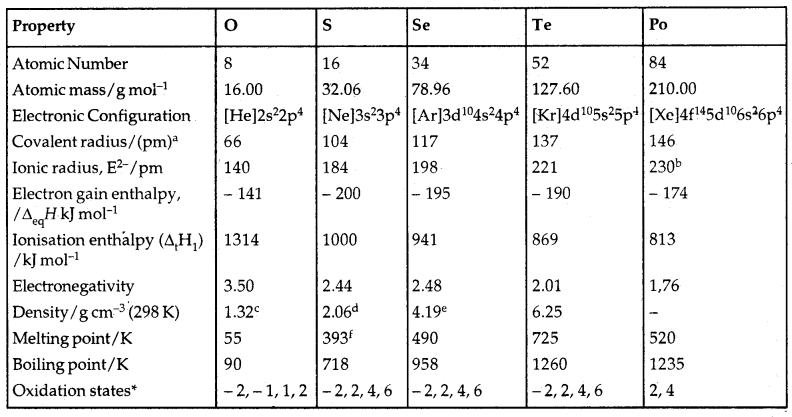
aSingle bond; bApproximate value; cAt the melting point; dRhombic sulphur; eHexagonal grey; fMonoclinic form, 673 K.
Oxygen shows oxidation states of + 2 and +1 in oxygen fluorides OF2 and O2F2 respectively.
→ Electron Gain Enthalpy: Because of the compact nature of the oxygen atom, it has less negative electron gain enthalpy than sulphur.
→ Electronegativity: Next to F, oxygen has the maximum value of electronegativity value amongst the elements. Within the group, its value decreases from top to bottom implying that metallic character increases from O to Po.
→ Physical Properties: Oxygen and Sulphur are non-metals, Selenium and Tellurium metalloids, whereas Polonium is a metal. Pollonium is radioactive [t1/2 = 13.8 days]
All these elements exhibit allotropy.
M.Pts and B.Pts. increase with the increase in atomic no. down the group. The large difference between the M.Pts and B.Pts. of oxygen and sulphur may be explained on the basis of their atomicity: Oxygen exists as diatomic molecules (O2) whereas Sulphur exists as polyatomic (S8).
Chemical Properties:
Oxidation states and trends in chemical reactivity:
O = – 2, -1, +1, (in O2F2), + 2 (in OF2)
S = – 2, + 2, +4, + 6 (in SF6)
Se = – 2, (+ 2), +4, + 6 (SeF6)
Te=-2,(+2), +4, + 6(TeF6)
Po = + 2, + 4
[Within brackets less stable.]
The stability of + 4 oxidation state increases and that of + 6 decreases due to the Inert-pair Effect. Bonding in + 4, + 6 oxidation states are primarily covalent.
The anomalous behaviour of oxygen is due to its
- small size,
- high electronegativity,
- absence of d-orbitals.
1. Reactivity with Hydrogen.
(a) There is strong hydrogen bonding in H2O which is not found in H2S.
(b) All the elements of Group 16 form hydrides of the type H2E[E = S, Se, Te, Po]
(c) H2O is neutral. Other hydrides are acidic and acidic character increases.
H2O < H2S < H2Se < H2Te < H2Po
(d) Thermal stability decreases:
H2O > H2S > H2Se > H2Te > H2Po
(e) H2O does do not have reducing character. Reducing character increases from H2S < H2Se < H2Te.
2. Reactivity with Oxygen: All these elements form oxides of EO2 and EO3 types [E = S, Se, Te, or Po]. Both types are acidic in nature.
3. Reactivity towards halogens: Elements of Group 16 form a large no. of halides of the type, EX6, EX4, EX2 where X = halogen. The stability of the halides decreases in the order F– > Cl > Br > I–
Dioxygen, O2:
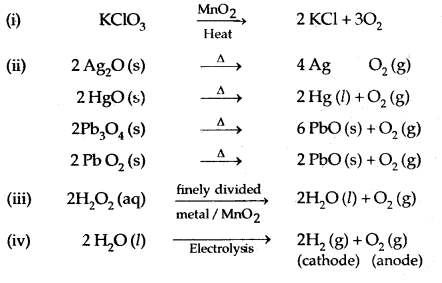
Industrially, O2 is obtained from air by first removing CO2 and water vapour and then, the remaining gases are liquified and fractionally distilled to give N2 and O2.
Properties of O2:
- Colourless, odourless gas.
- 30.8 cm33 of O2 soluble per litre of water at 293 K.
- It has three isotopes 16O, 17O, 18O.
- Molecular oxygen (O2) is paramagnetic.
- Reactions with metals, non-metals and other compounds are as follows:
Uses:
- It is used in oxyacetylene welding.
- Manufacture of many metals, particularly steel.
- Oxygen cylinders are widely used in hospitals, high altitude flying and mountaineering.
- The contraction of fuels, e.g., hydrazines in liquid oxygen, provides tremendous thrust in rockets.
→ Simple Oxides: A binary compound of oxygen with another element is called oxide.
Oxides can be simple like MgO, Al2O3 or mixed like Pb3O4, Pe3O4.
In general metallic oxides like CaO, BaO, Na2O are basic. Non-metal oxides like SO2, CO2 are acidic.
Some oxides like Al2O3 are amphoteric. They react with acids as well as bases.
Al2O3 + 6 HCl → 2AlCl3 + 3H2O.
Al2O3 + 2NaOH → 2Na AlO2 + H2O
Ozone, O3: Ozone is an allotropic form of oxygen. At a height of 20 km from sear level, it is formed from atmospheric oxygen in the presence of sunlight.
![]()
The ozone layer protects the earth’s surface from UV rays.
Preparation:
![]()
ΔH° = + 142 kJ mol-1
Properties:
- It is a pale blue gas, dark blue liquid and violet-black solid.
- O3 is thermodynamically unstable w.r.t. O2.
∴ It acts as a powerful oxidizing agent.
O3 → O2 + O
1. It oxidizes lead sulphide to lead sulphate.
PbS + 4O3(g) → PbSO4 (s) + 4O2
2. It oxidizes I– ions to iodine
2I– (aq) + H2O (l) + O3 (g) → 2 OH– (aq) + I2 (s) + O2 (g)
3. It oxidizes NO to NO2
NO (g) + O3 (g) → NO2 (g) + O2 (g)
Structure: The two oxygen-oxygen bond lengths in the ozone molecule are identical (128 pm) and the molecule is angular as expected with a bond angle of about 117°. It is a resonance hybrid of two main forms:
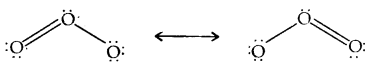
Uses:
- It is used as a germicide, disinfectant and for sterilising water.
- It is also used for bleaching oils, ivory, flour, starch.
- It is an oxidizing agent.
Sulphur-Allotropic Forms: Out of numerous allotropes, the two most important forms are:
- Yellow rhombic (α-sulphur),
- Monoclinic (β-Sulphur)
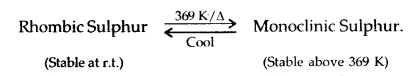
The temperature 369 K is called Transition temperature.
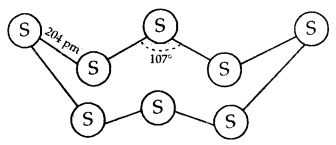
The structure of (a) S8 ring in rhombic sulphur and (b) S6 form:
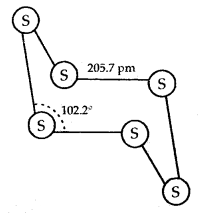
Both rhombic and monoClinic Sulphur have S8 molecules. These S8 molecules are packed to give different crystal structures. The S8 ring in both forms is puckered and has a crown shape.
In cyclo-S6 the ring adopts the chair form. At elevated temperatures (~ 1000 K) S2 is the dominant species and is paramagnetic like O2
Sulphur Dioxide (SO2):
Preparation:
1. By burning S in air or O2.
S(s) + O2(g) → SO2(g)
2. Lab. Method: By treating a sulphite with dilute H2SO44.
SO32- (aq) + 2H+ (aq) → H2O (l) + SO2(g)
3. Industrially, it is produced by roasting of sulphide ores:
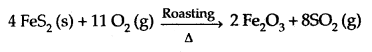
Properties:
1. It is a colourless gas with a pungent smell.
2. It is highly soluble in water.
3. It liquefies at room temp, under a pressure of 2 atmospheres.
4. Liquified SO2 boils at 263 K.
5, SO2 when dissolved in water, forms sulphurous acid.
SO2(g) + H2O (l) → H2SO3 (aq)
6. Reaction with NaOH:
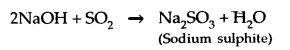
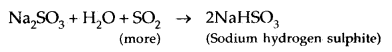
7. Reaction with Cl2:

8. Reaction with O2:
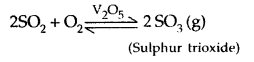
9. Moist SO2 behaves as a reducing agent. It reduces iron (III) ions to iron (II) ions and decolourised acidified potassium permanganate(VII) solution. It is a test for SO2 gas.
2Fe3+ (aq) +SO2 + 2H2O → 2 Fe2+ (aq) + SO42- + 4H+
5 SO2 + 2 MnO4– + 2H2O → 5 SO4 2- + 4H+ + 2 Mn2+
Structure: The molecule of SO2 is angular. It is a resonance hybrid of the two canonical structures
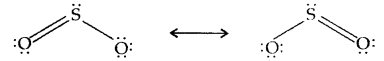
Uses:
- Refining of petroleum, sugar
- Bleaching wool and silk.
- Antichlor, disinfectant and preservative.
- Manufacture of H2S04 and other industrial chemicals.
- Liquid S02 is used as a solvent.
Oxo-Acids of Sulphur: Sulphur forms a number of oxo-acids such as H2SO2, H2S2O3, H2S2O4, H2S2O5, H2SxO6 (X = 2 to 5), H2SO4, H2S2O7, H2SO5, H2S2O8.
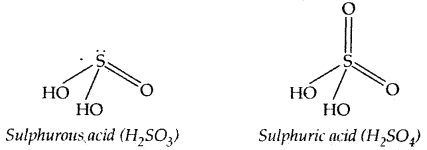
Structures of Sulpiwrous and Sulphuric acids
Sulphuric Acid (H2SO4): It is called the king of chemicals and is one of the most widely used industrial chemicals.
→ Manufacture: Sulphuric acid is manufactured by Contact Process which involves 3 steps:
- Burning of Sulphur or Sulphide ores in the air to produce SO2.
- Conversion of SO2 into SO3 by reaction with air in the presence of catalyst V2O5.
- Absorption of SO3, in H2SO4, to give Oleum (H2S2O7).
The key step is the catalytical oxidation of SO2 with O2 to give SO3 in the presence of V2O5.
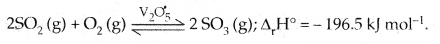
The reaction is exothermic, reversible and proceeds with a decrease in volume.
∴ Low temperature (720 K) and high pressure (2 bar) are favourable conditions for maximum yield.
The tempi should not be very low otherwise the rate of reaction will below.
The SO3 gas from the catalytical converter is absorbed in concentrated H2SO4 to produced Oleum (H2S2O7) which on dilution with water gives H2SO4 of the desired concentration.
SO3 + H2SO4 → H2S2O7 (oleum)
H2S2O7 + H2O → 2 H2SO4
The sulphuric acid obtained by the contact process is 96-98% pure.
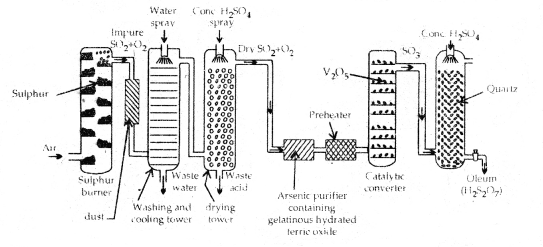
Flow diagram the iiai1ufat true of sulphuric acid
Properties:
- H2SO4 is a colourless, dense, oily liquid.
- It dissolves in water producing a large amount of heat. Hence it should be slowly added to water (and never water in acid)
The chemical properties of H2SO4 are due to its
(a) low volatility,
(b) strong acidic character,
(c) strong affinity for water,
(d) ability to act as an oxidizing agent.
In water, it ionises in 2 steps:
1. H2SO4 (aq) + H2O (l) → H3O+ (aq) + HSO4– (aq)
Ka1 = very large (> 10)
2. HSO4–(aq) + H2O (l) → H3O+ (aq) + SO4 2- (aq);
Ka2 = 1.2 × 10-2.
(a) It is used to prepare more volatile acids:
NaNO3 + H2SO4 → NaHSO4 + HNO3.
2 NaCl + H2SO4 → Na2SO4 + 2 HCl
(b) Cone. H2SO4 is a strong dehydrating agent
![]()
(c) Hot cone. H2SO4 is a strong oxidising agent
H2SO4 → H2O + SO2 + O
(d) Reaction with metals and non-metals:
Cu + 2 H2SO4 → CuSO4 + SO2 + 2H2O
C + 2H2SO4 (cone.) → CO2 + 2SO2 + 2H2O
Uses of H2SO2 :
- It is used in the manufacture of hundreds of other compounds and also in many industrial processes.
- Manufacture of fertilizers.
- Petroleum refining and manufacture of pigments, paints and dyestuff intermediates.
- Detergent industry.
- Metallurgical applications.
- Storage batteries and so on.
Group 17 Elements: Fluorine, chlorine, .bromine, iodine and astatine are the members of Group 17. They are collectively called halogens (sea-salt producers)
→ Occurrence: Fluorine is present as CaF2 (fluorspar) and Na3 AlF6 (cryolite). Seawater contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium. The deposits of dried up seas contain NaCl and carnallite, KCl. MgCl2,6Fl2O. Seaweeds contain 0.5% of iodine and Chile saltpetre contains up to 0.2% of sodium iodate.
→ Electronic Configuration: All these elements have seven electrons in their valence shell [ns2np3] which is 1 electron less than the next noble gas.
→ Atomic and ionic radii: The halogens have the smallest atomic radii in their respective periods due to the maximum effective nuclear charge. The size of the anions is greater than the corresponding atoms. The atomic and ionic radii increase from top to bottom.
→ Ionisation Enthalpy: They have little tendency to lose electrons. Thus they have very high ionisation enthalpy. I.E. decreases from top to bottom.
→ Electronegativity: They have high electronegativities. It decreases from top to bottom. Fluorine is the most electronegative element in the periodic table. [F = 4.0]
→ Electron Gain Enthalpy: Halogens have maximum negative- electron gain enthalpy in their corresponding periods. It becomes less negative down the group. Electron gain enthalpy of F is less than that of Cl due to the compact size of F.
→ Physical Properties: F2 and Cl2 are gases. Br2 is a liquid and I2 is solid. They are all diatomic. M.pts and B.pts increase from top to bottom. F2 is yellow, Cl2 is greenish-yellow, Br2 is red and I2 violet in colour.
F2 has a smaller enthalpy of dissociation as compared to Cl2.
→ Chemical Properties:
Oxidation States and Trends in Chemical Reactivity: All the halogens exhibit a -1 oxidation state. However, chlorine, bromine and iodine exhibit +1, + 3, +5 and +7 oxidation states also as explained below:
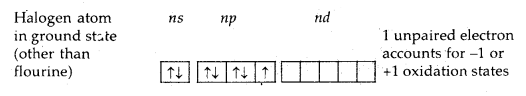
Table: Atomic and Physical Properties of Halogens (Group 17)
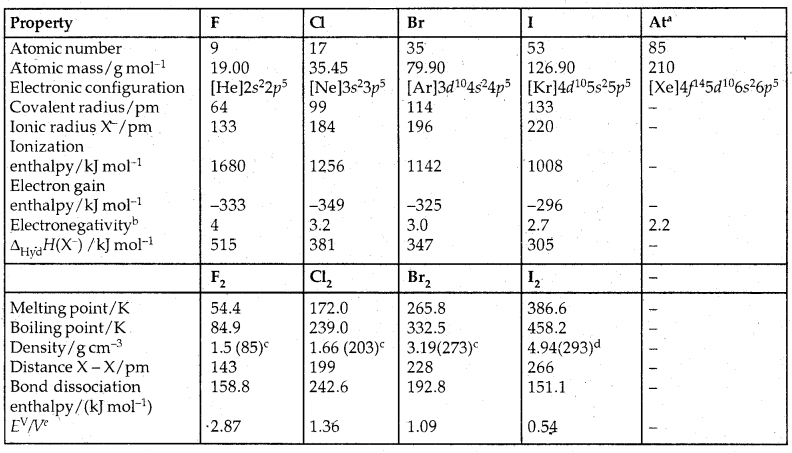
aRadioactive; bPauling scale; cFor the liquid at temperatures (K) given in the parentheses; dsolid; eThe half-cell reaction is X2(g) + 2e– → 2X–(aq).
The higher oxidation states of chlorine, bromine and iodine are realised mainly when the halogens are in combination with the small and highly electronegative fluorine and oxygen atoms, e.g., in interhalogens, oxides and oxoacids.
The oxidation states of +4 and +6 occur in the oxides and oxoacids of chlorine and bromine. The fluorine atom has no d-orbitals in its valence shell and therefore cannot expand its octet. Being the most electronegative, it exhibits only a -1 oxidation state.
All the halogens are highly reactive. They react with metals and non-metals to form halides. The reactivity of the halogens decreases down the group.
The ready acceptance of an electron is the reason for the strong oxidising nature of halogens. F2 is the strongest oxidising halogen and it oxidises other halide ions in solution or even in the solid phase.
In general, a halogen oxidises halide ions of higher atomic number.
F2 + 2X– → 2F + X2 (X = Cl, Br or I)
Cl– + 2X– → 2CT + X2 (X = Br or I)
Br2 + 2I– → 2Br + I2
The decreasing oxidising ability of the halogens in aqueous solution down the group is evident from their standard electrode potentials which are dependent on the parameters indicated below:
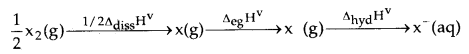
The relative oxidising power of halogens can further be illustrated by their reactions with water. Fluorine oxidises water to oxygen whereas chlorine and bromine react with water to form corresponding hydrohalic and hypohalous acids.
The reaction of iodine with water is non-spontaneous. In fact, I– can be oxidised by oxygen in an acidic medium; just the reverse of the reaction observed with fluorine.
2F2 (g) + 2 H2O (l) → 4H+ (aq) + 4 F (aq) + O2 (g)
X2 (g) + H2O (l) → HX (aq) + HOX (aq) (where X = Cl or Br)
4I– (aq) + 4H+ (aq) + O2 (g) → 2I2 (s) + 2 H2O (l)
Anomalous behaviour of Fluorine: It is due to
- Highest electronegativity and Ionisation enthalpy in Group 17.
- Small size.
- Non-availability of d-orbitals.
- The low bond dissociation energy of the F-F bond.
1. Reactivity towards hydrogen: They all react with hydrogen to give hydrogen halides, but an affinity for hydrogen decreases from F to I.
The acidic strength is in order HF < HCl < HBr < HI and stability is under HF > HCl > HBr > HI.
2. Reactivity towards oxygen: Halogens form many oxides with oxygen but most of them are unstable. F forms OF2 and O2F2, but only OF2 is stable. Oxides of Cl, Br, I am powerful oxidizing agents.
3. Reactivity towards metals
Mg + Br2 (l) → MgBr2(s)
The ionic character of halids decreases in the order MF > MCI > MBr > MI.
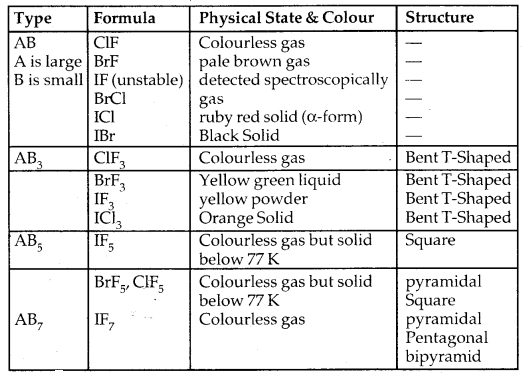
4. Formation of interhalogen compounds: They form interhalogen compounds among themselves of the type AB, AB3, AB5, AB7 where A = larger size halogen and B = smaller size halogen.
Chlorine, Cl2:
Preparation:
1. By heating MnO2 with cone. HCl
![]()
However a mixture of common salt and cone. H2SO4 is used in place of HCl.
2 NaCl + MnO2 + 3 H2SO4 → 2 NaHSO4 + MnSO4 + 2H2O + Cl2
2. By the action of HC1 on KMn04
2 KMnO4 + 16HCl → 2 KCl + 2 MnCl2 + 5 Cl2 + 8 H2O
Manufacture of Cl2:
1. Deacon’s process:
![]()
2. Cl2 is obtained by the electrolysis of blue solution.
Properties:
1. Greenish-yellow gas with a pungent smell.
2. 2.5 times heavier than air.
3. Can be liquified easily into greenish-yellow liquid.
4. Soluble in water.
5. It reacts with a number of metals and non-metals and other compounds.
2Al + 3Cl2 → 2Al Cl3
2 Na + Cl2 → 2NaCl
2 Fe +3 Cl2 → 2FeCl3
P4 +6 Cl2 → 4PCl
S3 +4 Cl2 → 4 S2Cl2
H2 + Cl2 → 2HCl
H2S + Cl2 → 2HCl + S
C10H16 + 8 Cl2 → 16HCl + 10 C.
8NH3 (excess) + Cl2 → 6NH4Cl + N2
NH2 + 3Cl2 (excess) → NCl3 + 3HCl
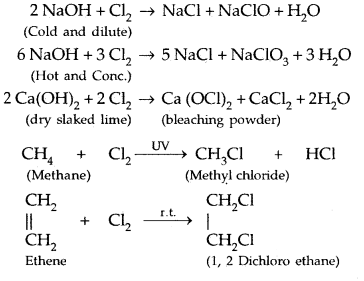
6. Chlorine dissolved in water on standing loses its yellow colour due to the formation of HCl and HOCl. Hypochlorous acid (HOCl) so formed, gives nascent oxygen which is responsible for oxidising and bleaching properties: Its oxidising properties are:
(a) 2 FeSO4 + H2SO4 + Cl2 → Fe2(SO4)3 + 2 HCl
Na2SO3 + Cl2 + H2O → 4 Na2SO4 + 2 HCl
SO2 + 2H2O + Cl2 → 4 H2SO4 + 2HCl
I2 + 6 H2O + 5 Cl2 → 2 HIO3 + 10 HCl.
(b) It is a powerful bleaching agent due to oxidation. It is permanent.
![]()
Uses:
- For bleaching wood pulp, cotton and textiles.
- In the extraction of gold and platinum.
- Manufacture of dyes, drugs, DDT, refrigerants etc.
- In sterilising water.
Hydrogen Chloride, HCl:
Preparation-Lab.
Method:
2 NaCl + H2SO2 → Na2SO4 + 2 HCl cone.
Properties:
- It is a colourless gas with a pungent smell.
- Highly soluble in water.
- Can be liquified easily to a colourless liquid and solidified to a white crystalline solid.
- It ionises in an aqueous solution.
HCl + H2O (l) → 4 H3O+ (aq) + Cl– (aq); Ka = 107
The high value of Ka shows it is a strong acid in water. - Reaction with NH3
NH3 + HCl → NH4Cl (White dense fumes) - 3 parts of cone. HCl and one part of cone. HNO3 on mixing give aqua regia which is used to dissolve, Au, Pt.
Au + 4H+ + NO3 + 4 Cl– → AUCl4– + NO + 2H2O
3Pt + 16H+ + 4NO3– + 18Cl– → 3PtCl62- + 4NO + 8H2O . - Reaction with salts:
Na2CO3 + 2HCl → 2 NaCl + H2O + CO2 ↑
NaHCO3 + HCl → NaCl + H2O + CO2 ↑
Na2SO3 + 2HCl → 2 NaCl + H2O + SO2 ↑
Uses of HCl:
- Manufacture of Cl2, NH4Cl and glucose from com starch.
- Extracting glue from bones and purifying bone black.
- In medicine and as a lab. reagent
Table: Oxo-Acids of Halogens
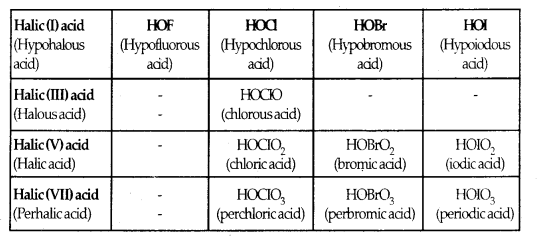
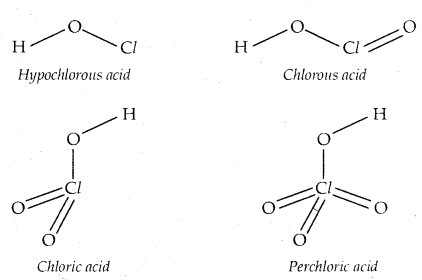
Structures of Oxoacids:
Inter Halogen Compounds:
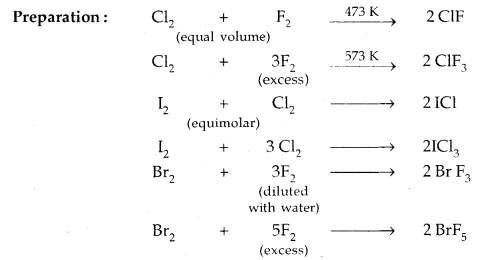
Some Properties of Interhalogen Compounds:
Uses:
- Interhafogen compounds are very useful fluorinating agents.
- They can be used as non-aqueous solvents.
- ClF3 and BrF3 are used for the production of UF6 in the enrichment of 236U.
U (s) + 3 ClF3 (l) → UF6(g) + 3 Cl F (g) argon (Ar)
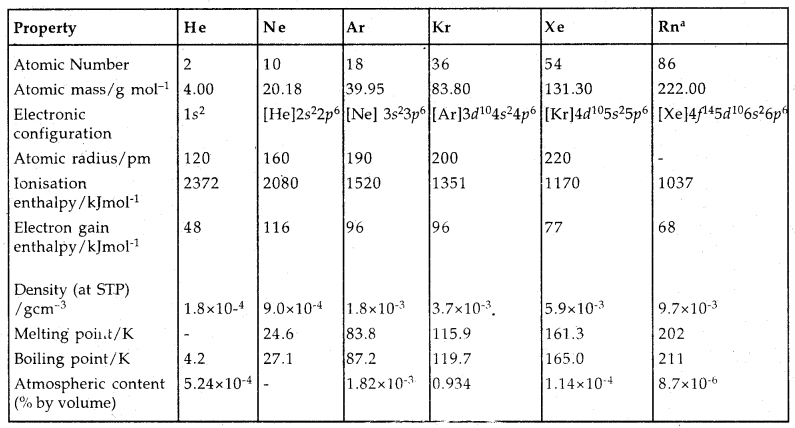
Group 18 Elements: Group 18 Elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn). Because of ns2nph structure [1s2 for He], They are chemically unreactive. They are called noble gases.
→ Occurrence: Except radon (Rn) which is radioactive, all noble gases occur in the atmosphere in traces. Noble gases constitute 1 % (by volume) of dry air. He is present in pitch blende, monazite, cleveite. Radon is obtained as a decay product of 226Ra.
22688Ra → 22286Ra + 42He
→ Electronic Configuration: All noble gases have ns2np6 electronic configuration except He which is 1s2.
→ Ionisation Enthalpy: They have very high I.E. which decreases down the group.
→ Atomic radii: They increase down the group with an increase in at. number.
→ Electron Gain Enthalpy: They have large positive values because of their stable electronic configuration. They have no tendency to accept electrons.
Physical Properties:
- These gases are monoatomic.
- They are colourless, odourless and tasteless.
- They are sparingly soluble in water.
- Due to weak dispersion forces, they have very low m.pts and b. pts.
- He has the lowest B.pt (4.2 K) of any known substance.
→ Chemical Properties: In general, noble gases are least reactive. It is due to
- Stable electronic configuration of ns2np6 (or 1s2 for He).
- They have high Ionisation enthalpy and more positive electron gain enthalpy,
Neil Bartlett prepared the first compound of Xe: red coloured Xe+ Pt F6– by mixing PtF6– and Xenon. The compounds of Kr are fewer. He, Ne and Ar are not found to form any true compounds. Only KrF2 has been studied. Compound involving Rn viz. RnF2 has been identified but not isolated.
Xenon-fluorine Compounds: Xenon forms three binary compounds with fluorine: XeF2, XeF4, XeF6 by the direct combination of elements under appropriate experimental conditions.
Xenon-oxygen compounds: Xenon and oxygen form XeO3. Partial hydrolysis of XeF4 and XeF6 gives oxyfluorides XeOF4 and XeO2F2.

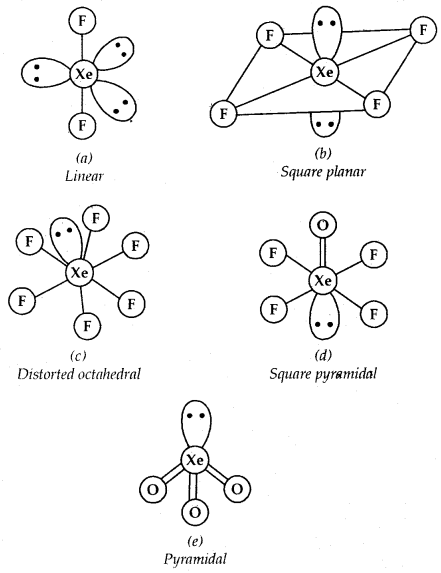
structures of. (a) XeF2, (b) XeF4, (c) XeF6, (d) XeOF4 and (e) XeO3
XeO3 is a colourless explosive solid and has a pyramidal molecular structure. XeOF4 is a colourless volatile liquid and has a square pyramidal molecular structure.
Uses: He is used in filling balloons because He is a light and non-inflammable gas. It is used in gas-cooled nuclear reactors. Liquid He is used in low temp, physics. It is used in the driving apparatus.
Neon is used in discharge tubes and fluorescent bulbs for display purposes. Argon is mainly used to provide an inert atmosphere in high-temperature metallurgical processes.
There are no significant uses of Xenon and Krypton. They are used in light bulbs designed for special purposes.