Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Coordination Compounds and Double Salts
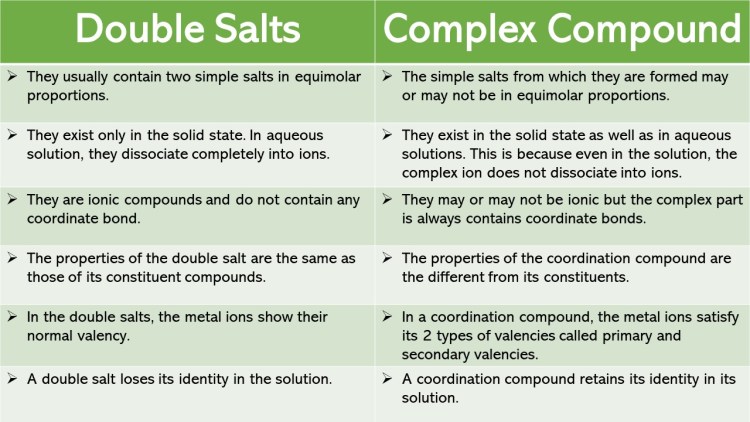
When two or more stable compounds in solution are mixed together and allowed to evaporate, in certain cases there is a possibility for the formation of double salts or coordination compounds. For example when an equimolar solution of ferrous sulphate and ammonium sulphate are mixed and allowed to crystallise, a double salt namely Mohr’s salt (Ferrous ammonium sulphate, FeSO4.(NH4)2SO4.6H2O) is formed.
Let us recall the blood red colour formation in the inorganic qualitative analysis of ferric ion, the reaction between ferric chloride and potassium thiocyanate solution gives a blood red coloured coordination compound, potassium ferrithiocyanate K3[Fe(SCN)6].
If we perform a qualitative analysis to identify the constituent ions present in both the compounds, Mohr’s salt answers the presence of Fe2+, NH4+ and SO42- ions, whereas the potassium ferrithiocyanate will not answer Fe3+ and SCN–ions. From this we can infer that the double salts lose their identity and dissociates into their constituent simple ions in solutions, whereas the complex ion in coordination compound, does not loose its identity and never dissociate to give simple ions.
Double salts and coordination compounds are complex compounds. The difference between double salt and coordination compound is that a double salt contains two salts with different crystal structures whereas a coordination compound contains a central metal ion surrounded by molecules or ions known as ligands.
A salt is essentially composed of an anion and a cation. But the main difference between a double salt and a complex salt is that a double salt is a combination of two salt compounds whereas a complex salt is a molecular structure that is composed of one or more complex ions.
A complex salt is a compound composed of a central metal atom having coordination bonds with ligands around it. Do not completely dissociate into its ions in water. It cannot be easily analyzed by determining the ions in the aqueous solution.
Both double salt as well sas complexes are formed by the combination of two or more stabel compounds in stoichiometic reatio. However they differ in the fact that double salt disssociate ito simple ions cmpletely with dissolved in water. However complex ions do not simple ions completely.
A double salt is a salt that contains more than one cation or more than one anion. Other examples include potassium sodium tartrate, ammonium iron (II) sulfate (Mohr’s salt), and bromlite. The fluorocarbonates contain fluoride and carbonate anions.
Double salts and coordination compounds are complex compounds. The difference between double salt and coordination compound is that a double salt contains two salts with different crystal structures whereas a coordination compound contains a central metal ion surrounded by molecules or ions known as ligands.
A double salt is formed from a three-component system, comprising two separate salts and water, and at a given temperature this may be represented by a triangular diagram. Phase diagram of a three-component system.
Double salts are addition compounds which lose their identity in aqueous solution whereas complexes which are also addition compounds do not lose their identity in aqueous solution.
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O).
A complex salt is a salt that contains one or more complex ions – ions with metal centers and different molecules attached. Complex salts include potassium ferricyanide (used to create dyes and in blueprint paper) and potassium argentocyanide (used in silver plating).
The molecularity of the chemical reaction is equal to the sum of the stochiometric coefficients of the reactants in the chemical equation of the reaction. It is also defined as the number of reactant molecules taking part in a single step of the reaction.