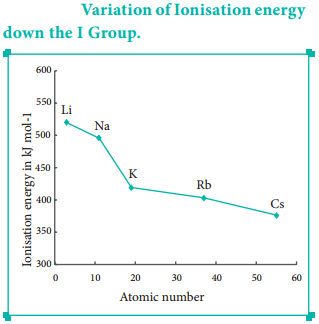
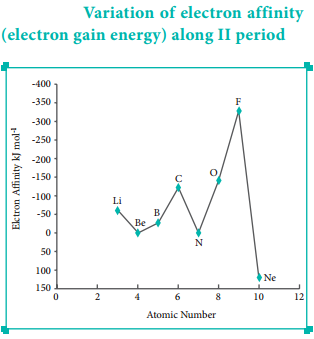
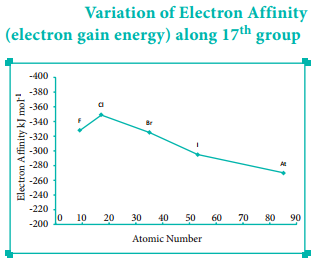
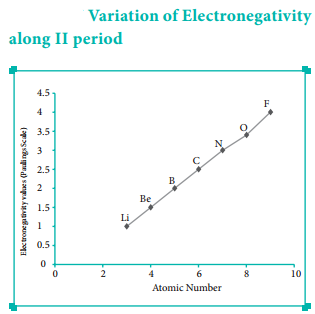
Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Hydrides
Hydrogen forms binary hydrides with many electropositive elements including metals and non-metals. It also forms ternary hydrides with two metals. E.g., LiBH4 and LiAlH4. The hydrides are classified as ionic, covalent and metallic hydrides according to the nature of bonding. Hydrides formed with elements having lower electronegativity than hydrogen are often ionic, whereas with elements having higher electronegativity than hydrogen form covalent hydrides.
Ionic (Saline) Hydrides:
These are hydrides composed of an electropositive metal, generally, an alkali or alkaline-earth metal, except beryllium and magnesium, formed by transfer of electrons from metal to hydrogen atoms. They can be prepared by the reaction of elements at about 400°C. These are salt-like, high-melting, white crystalline solids having hydride ions (H–) and metal cations (Mn+).
2 Li + H2 → 2 LiH
2 Ca + 2H2 → 2 CaH2
Covalent (Molecular) Hydrides:
They are compounds in which hydrogen is attached to another element by sharing of electrons. The most common examples of covalent hydrides of non-metals are methane, ammonia, water and hydrogen chloride. Covalent hydrides are further divided into three categories, viz., electron precise (CH4, C2H6, SiH4, GeH4), electrondeficient (B2H6) and electron-rich hydrides (NH3, H2O). Since most of the covalent hydrides consist of discrete, small molecules that have relatively weak intermolecular forces, they are generally gases or volatile liquids.
Metallic (Interstitial) Hydrides:
Metallic hydrides are usually obtained by hydrogenation of metals and alloys in which hydrogen occupies the interstitial sites (voids). Hence, they are called interstitial hydrides; the hydrides show properties similar to parent metals and hence they are also known as metallic hydrides.
Most of the hydrides are non-stoichiometric with variable composition (TiH1.5-1.8 and PdH0.6-0.8), some are relatively light, inexpensive and thermally unstable which make them useful for hydrogen storage applications. Electropositive metals and some other metals form hydrides with the stoichiometry MH or sometimes MH2 (M = Ti, Zr, Hf, V, Zn).