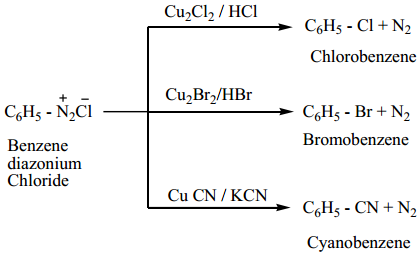
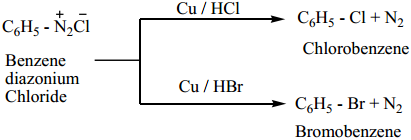
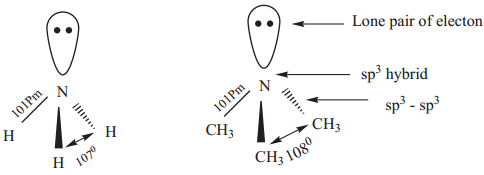
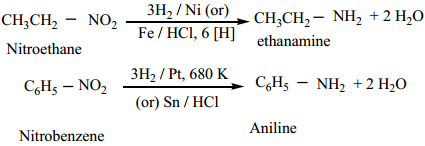
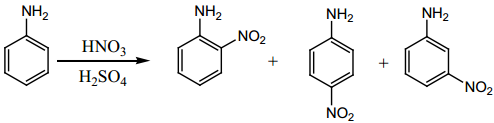
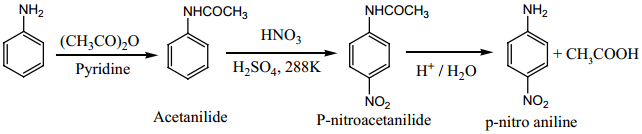
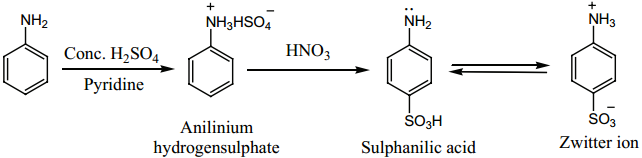
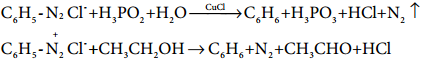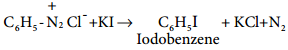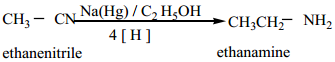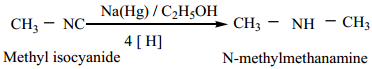Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Food Additives of Chemistry In Everday Life
Have you ever noticed the ingredients that is printed on the cover of the packed food materials such as biscuits, chocolates etc… You might have noticed that emulsifiers such as 322, 472E, dough conditioners 223 etc… are used in the preparation, in addition to the main ingredients such as wheat flour, edible oil, sugar, milk solid etc… Do you think that these substances are necessary? Yes. These substances enhance the nutritive, sensory and practical value of the food.
They also increase the shelf life of food. The substances which are not naturally a part of the food and added to improve the quality of food are called food additives.
Important Categories of Food Additives
- Aroma compounds
- Food colours
- Preservatives
- Stabilizers
- Artificial Sweeteners
- Antioxidants
- Buffering substances
- Vitamins and minerals
Advantages of Food Additives:
- Uses of preservatives reduce the product spoilage and extend the shelf-life of food
- Addition of vitamins and minerals reduces the mall nutrient
- Flavouring agents enhance the aroma of the food
- Antioxidants prevent the formation of potentially toxic oxidation products of lipids and other food constituents
Preservatives:
Preservatives are capable of inhibiting, retarding or arresting the process of fermentation, acidification or other decomposition of food by growth of microorganisms. Organic acids such as benzoic acid, sorbic acid and their salts are potent inhibitors of a number of fungi, yeast and bacteria. Alkyl esters of hydroxy benzoic acid are very effective in less acidic conditions. Acetic acid is used mainly as a preservative for the preparation of pickles and for preserved vegetables.
Sodium metasulphite is used as preservatives for fresh vegetables and fruits. Sucrose esters with palmitic and steric acid are used as emulsifiers. In addition that some organic acids and their salts are used as preservatives. In addition to chemical treatment, physical methods such as heat treatment (pasteurisation and sterilisations), cold treatment (chilling and freezing) drying (dehydration) and irradiation are used to preserve food.
Antioxidants:
Antioxidants are substances which retard the oxidative deteriorations of food. Food containing fats and oils is easily oxidised and turn rancid. To prevent the oxidation of the fats and oils, chemical BHT (butylhydroxy toluene), BHA(Butylated hydroxy anisole) are added as food additives.
They are generally called antioxidants. These materials readily undergo oxidation by reacting with free radicals generated by the oxidation of oils, thereby stop the chain reaction of oxidation of food. Sulphur dioxide and sulphites are also used as food additives. They act as anti-microbial agents, antioxidants and enzyme inhibitors.
Sugar Substituents:
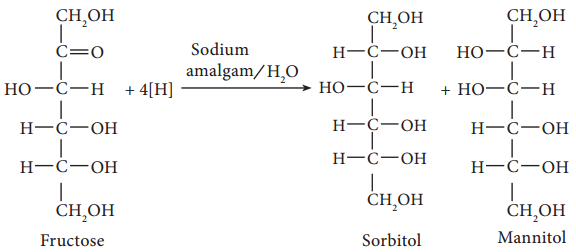
These compounds that are used like sugars (glucose, sucrose) for sweetening, but are metabolised without the influence of insulin are called sugar substituents. Eg. Sorbitol, Xylitol, Mannitol.
Artificial Sweetening Agents:
Synthetic compounds which imprint a sweet sensation and possess no or negligible nutritional value are called artificial sweeteners. Eg. Saccharin, Aspartame, sucralose, alitame etc…