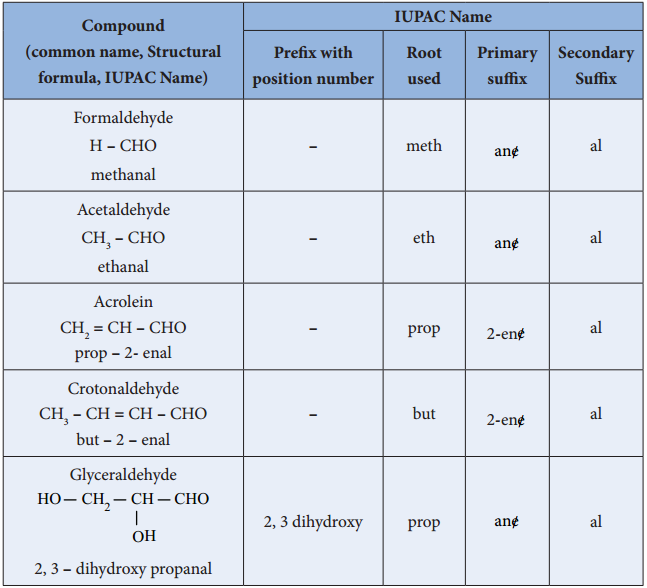
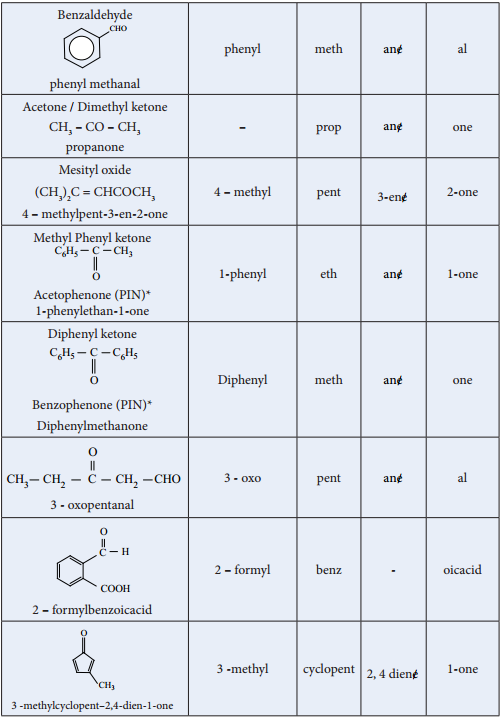
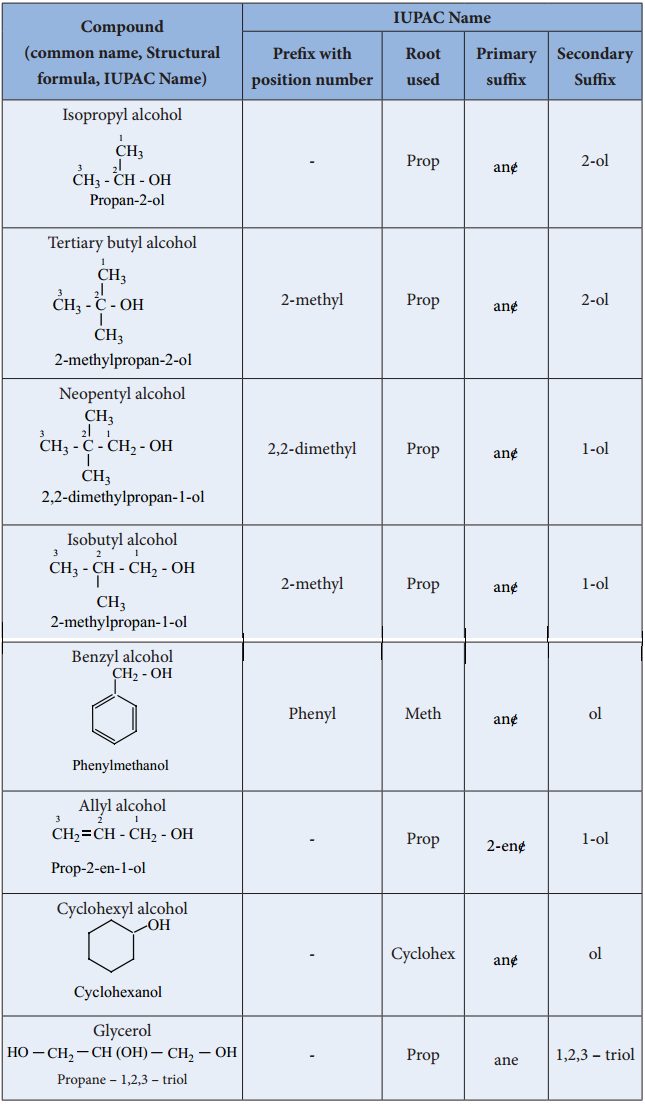
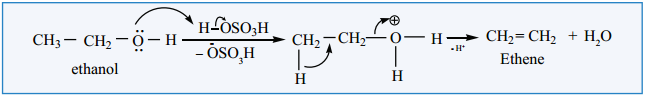
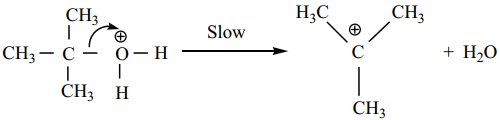
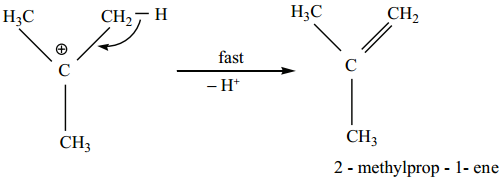
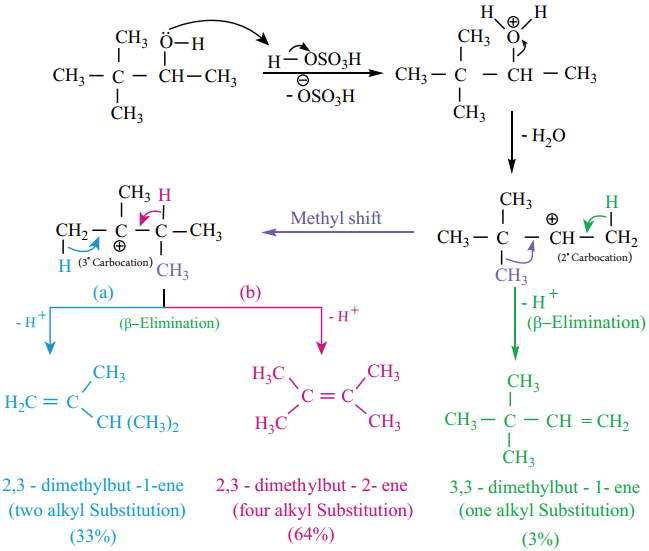
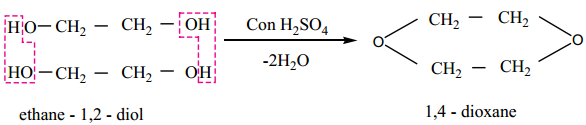
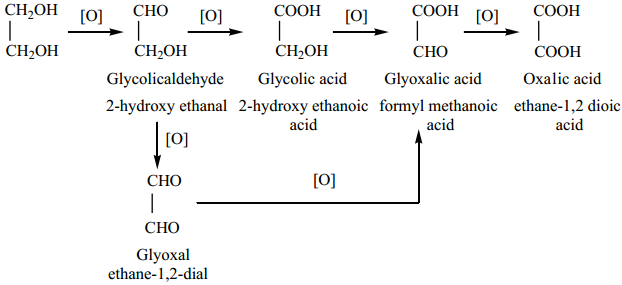
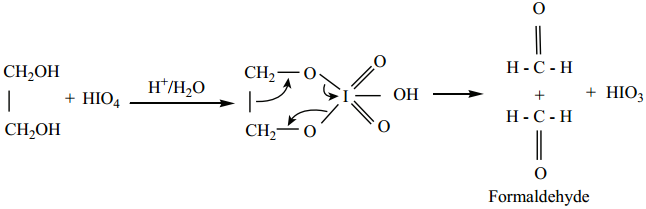
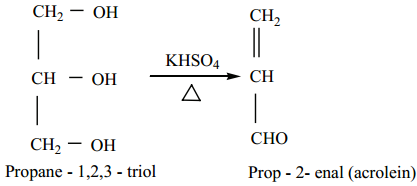
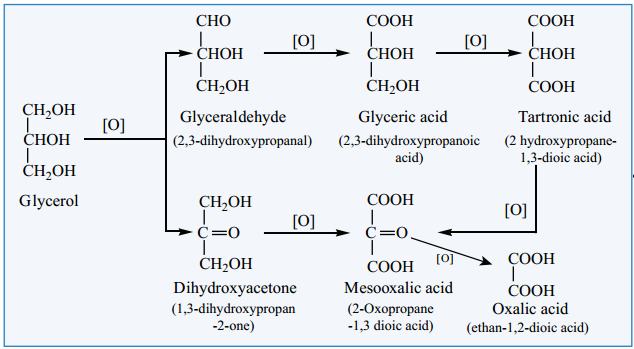
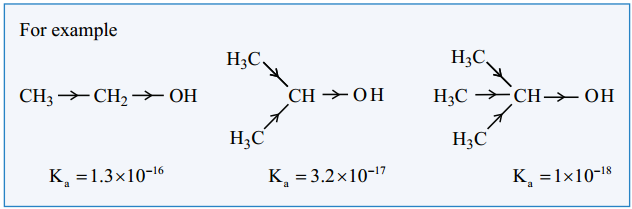
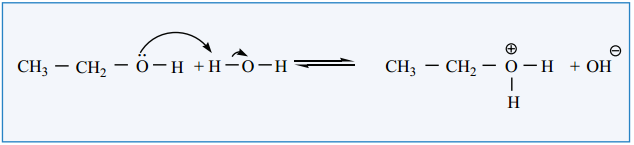
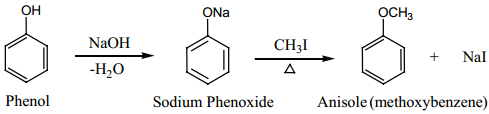
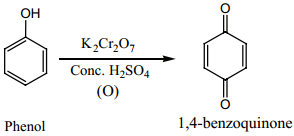
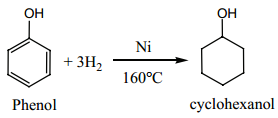
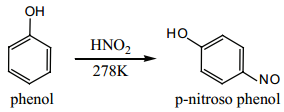
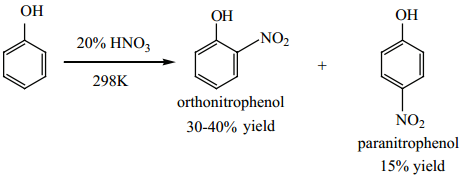
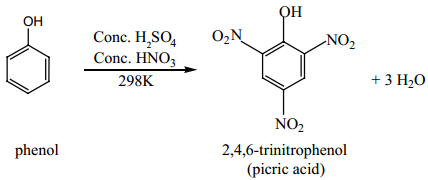
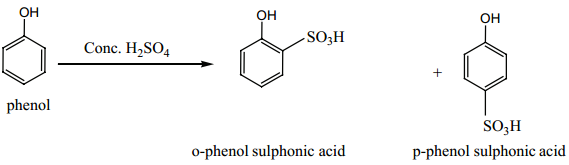
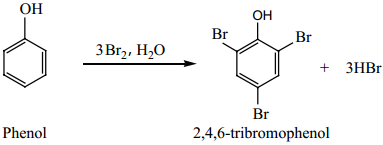
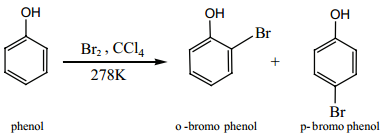
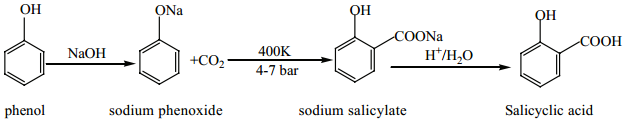
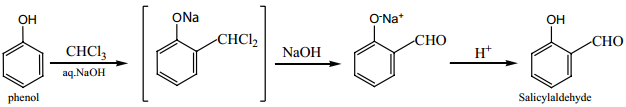
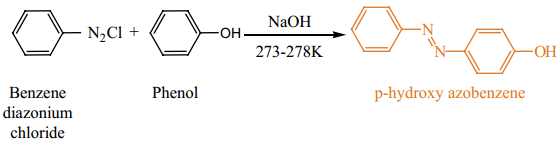
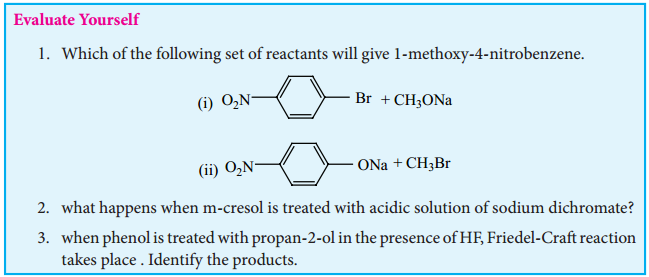
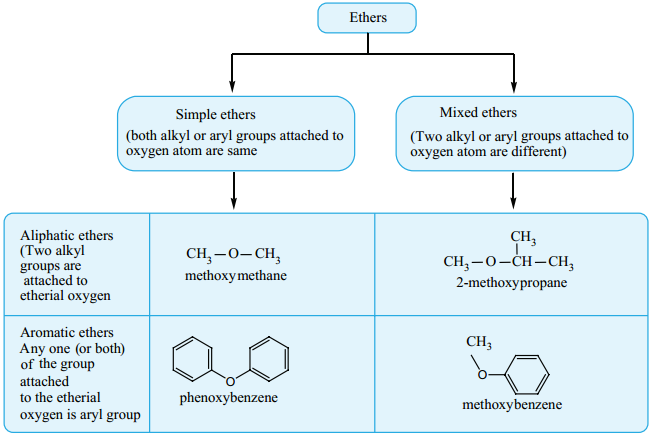
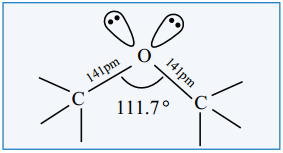
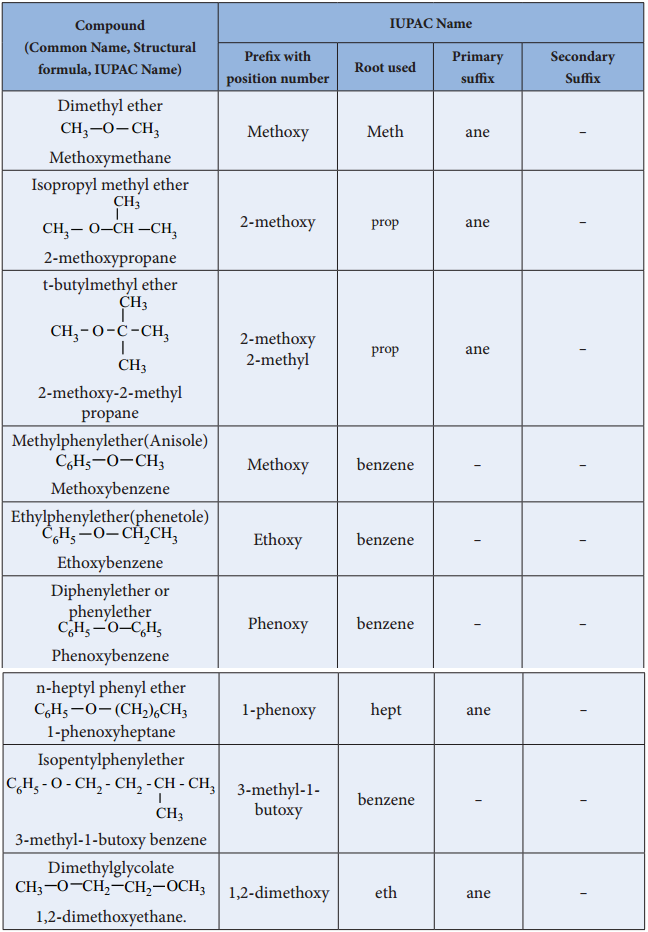
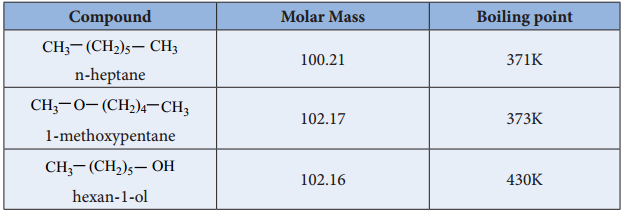
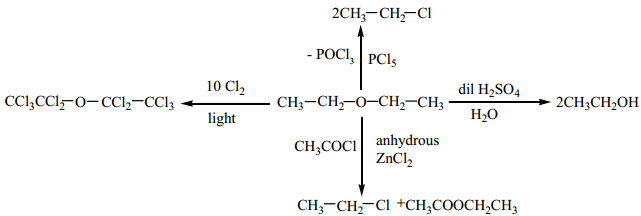
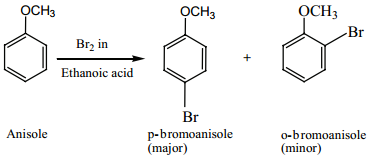
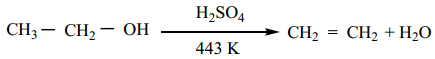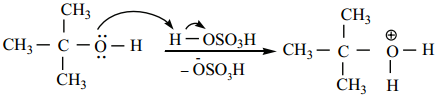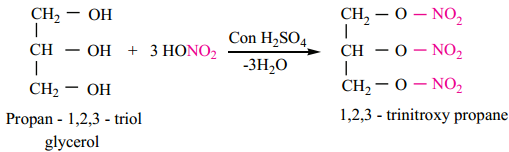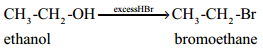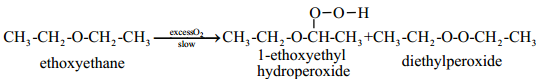Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Chemical Properties of Aldehydes and Ketones
A. Nucleophilic Addition Reactions
This reaction is the most common reactions of aldehydes and ketones. The carbonyl carbon carries a small degree of positive charge. Nucleophile such as CN– can attack the carbonyl carbon and uses its lone pair to form a new carbon – nucleophile ‘σ’ bond, at the same time two electrons from the carbon – oxygen double bond move to the most electronegative oxygen atom. This results in the formation of an alkoxide ion. In this process, the hybridisation of carbon changes from sp2 to sp3.
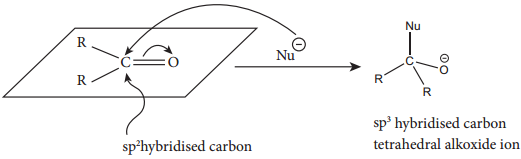
The tetrahedral intermediate can be protonated by water or an acid to form an alcohol.
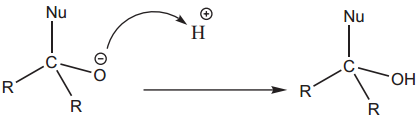
In general, aldehydes are more reactive than ketones towards nucleophilic addition reactions due to +I and steric effect of alkyl groups.
Examples
1. Addition of HCN
Attack of CN– on carbonyl carbon followed by protonation gives cyanohydrins.
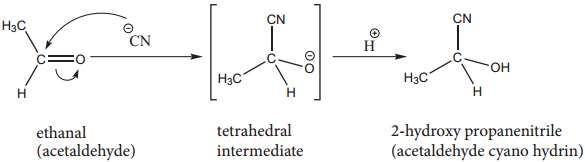
The cyanohydrins can be converted into hydroxy acid by acid hydrolysis. Reduction of cyanohydrins gives hydroxy amines.
2. Addition of NaHSO3
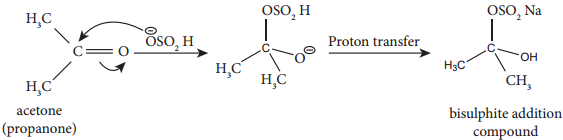
This reaction finds application in the separation and purification of carbonyl compound. The bisulphate addition compound is water soluble and the solution is treated with mineral acid to regenerate the carbonyl compounds.
3. Addition of Alcohol
When aldehydes / ketones is treated with 2 equivalents of an alcohol in the presence of an acid catalyst to form acetals.
Example
When acetaldehyde is treated with 2 equivalent of methanol in presence of HCl, 1, 1, – dimethoxy ethane is obtained.
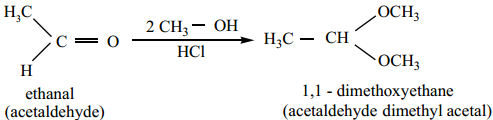
Mechanism
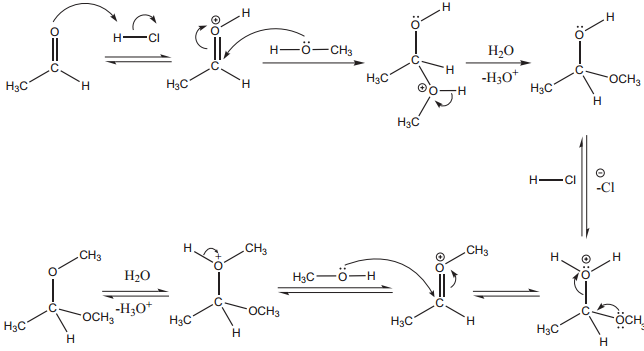
4. Addition of Ammonia and its Derivatives
When the nucleophiles, such as ammonia and its derivative image 6 is treated with carbonyl compound, nuceophilic addition takes place, the carbonyl oxygen atom is protonated and then elimination takes place to form carbon – nitrogen double bond image 7
When G – alkyl, aryl, OH, NH2, C6H5NH, NHCONH2 etc…
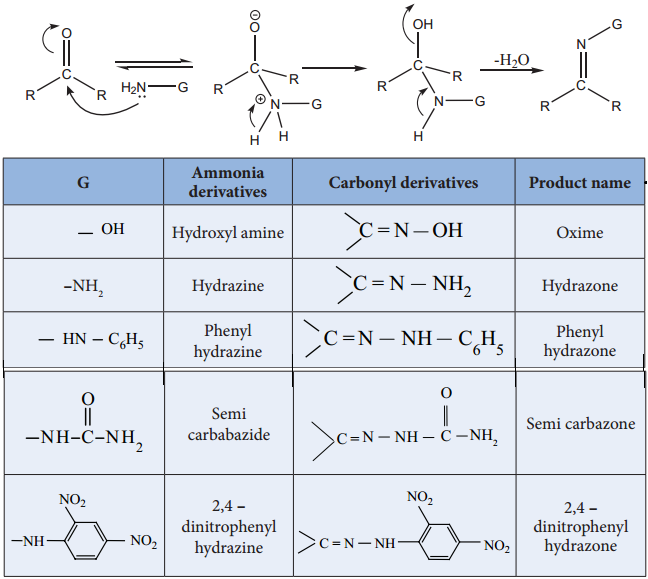
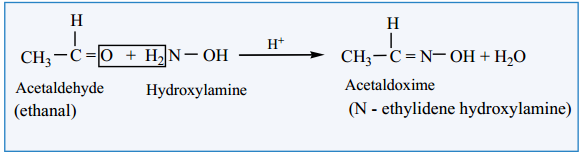
(i) Reaction with Hydroxyl Amine
Aldehyde and ketones react with hydroxylamine to form oxime.
Example:
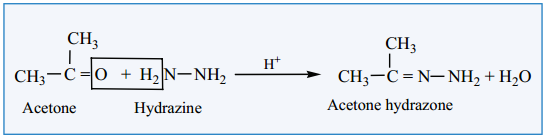
(ii) Reaction with Hydrazine
Aldehydes and ketones react with hydrazine to form hydrazone.
Example:
(iii) Reaction with Phenyl Hydrazine
Aldehydes and ketones react with phenyl hydrazine to form phenyl hydrazone.
Example:
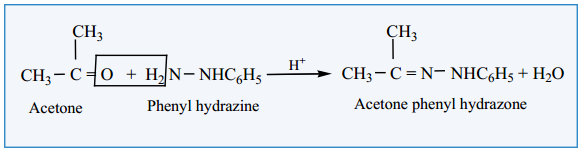
5. Reaction with NH3
(i) Aliphatic aldehydes (except formaldehyde) react with an ethereal solution of ammonia to form aldimines.
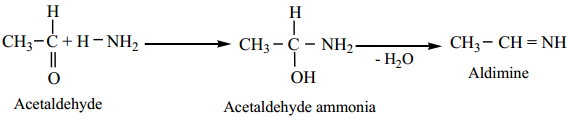
(ii) Formaldehyde reacts with ammonia to form hexa methylene tetramine, which is also known as Urotropine.
Structure
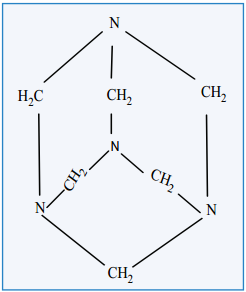
Uses
1. Urotropine is used as a medicine to treat urinary infection.
2. Nitration of Urotropine under controlled condition gives an explosive RDX (Research and development explosive). It is also called cyclonite or cyclotri methylene trinitramine.
3. Acetone reacts with ammonia to form diacetone amine.
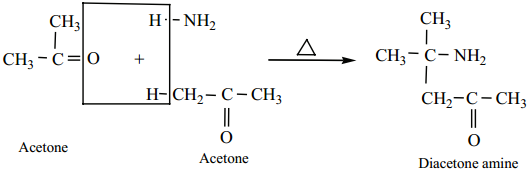
4. Benzaldehyde form a complex condensation product with ammonia.
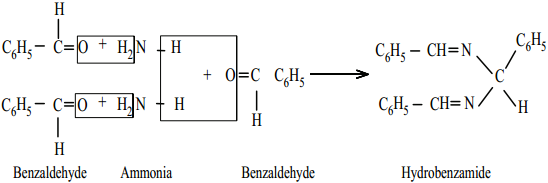
B. Oxidation of Aldehydes and Ketones
(a) Oxidation of Aldehydes
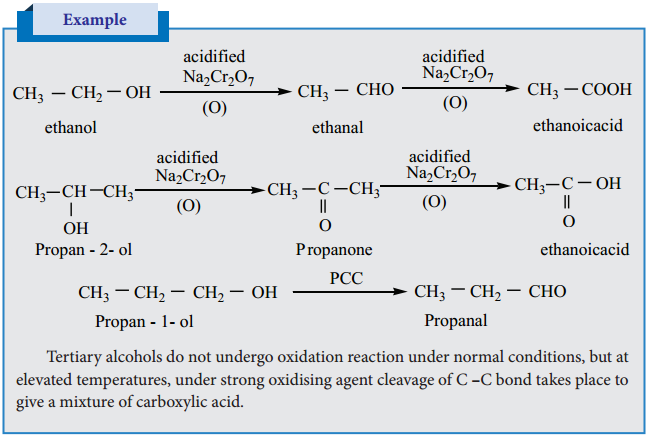
Aldehydes are easily oxidised to carboxylic acid containing the same number of carbon atom, as in parent aldehyde. The common oxidising agents are acidified K2Cr2O7, acidic or alkaline KMnO4 or chromic oxide.
Example
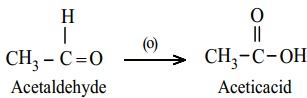
(b) Oxidation of Ketone
Ketones are not easily oxidised. Under drastic condition or with powerful oxidising agent like Con. HNO3, H+/KMnO4, H+/K2Cr2O7, cleavage of carbon-carbon bond takes place to give a mixture of carboxylic acids having less number of carbon atom than the parent ketone.
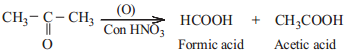
The oxidation of unsymmetrical ketones is governed by Popoff ’s rule. It states that during the oxidation of an unsymmetrical ketone, a (C-CO) bond is cleaved in such a way that the keto group stays with the smaller alkyl group.
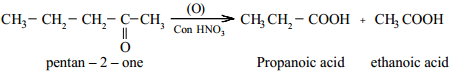
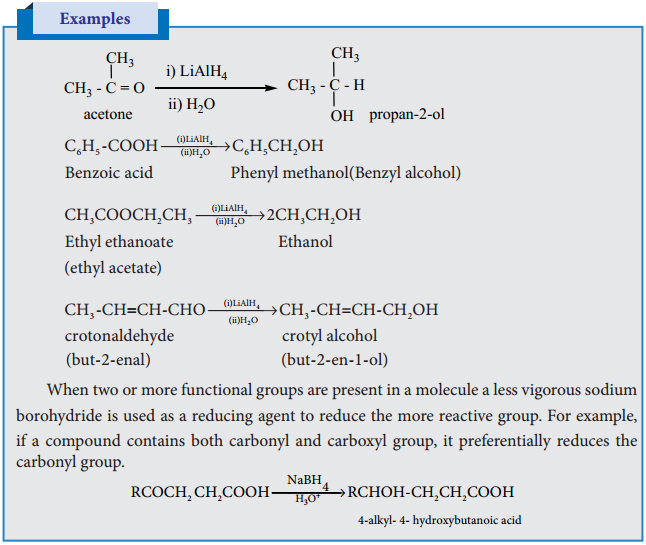
C. Reduction Reactions
(i) Reduction to Alcohols
We have already learnt that aldehydes and ketones can be easily reduced to primary and secondary alcohols respectively. The most commonly used reducing agents are Lithium Aluminium hydride (LiAlH4), and Sodium borohydride (NaBH4).
(a) Aldehyde are Reduced to Primary Alcohols
Example
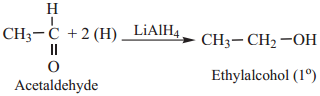
(b) Ketone are Reduced to Secondary Alcohols.
Example
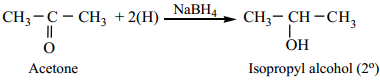
The above reactions can also be carried out with hydrogen in the presence of metal catalyst like Pt, Pd, or Ni. LiAlH4 and NaBH4 do not reduce isolated carbon – carbon double bonds and double bond of benzene rings. In case of α, β unsaturated aldehyde and ketones, LiAlH4 reduces only C = O group leaving C = C bond as such.
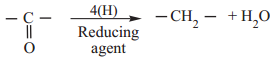
(ii) Reduction to Hydrocarbon
The carbonyl group of aldehydes and ketones can be reduced to methylene group using suitable reducing agents to give hydrocarbons.
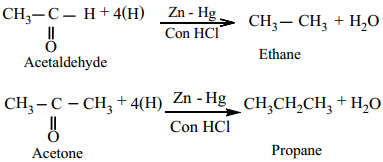
(a) Clemmensen Reduction
Aldehydes and Ketones when heated with zinc amalgam and concentrated hydrochloric acid gives hydrocarbons.
Example
(b) Wolf Kishner Reduction
Aldehydes and Ketones when heated with hydrazine (NH2NH2) and sodium ethoxide, hydrocarbons are formed Hydrazine acts as a reducing agent and sodium ethoxide as a catalyst.
Example
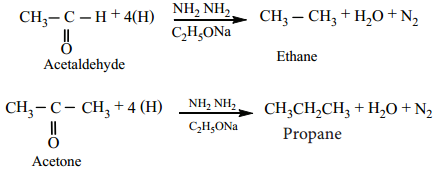
Aldehyde (or) ketones is first converted to its hydrazone which on heating with strong base gives hydrocarbons.
(iii) Reduction to Pinacols:
Ketones, on reduction with magnesium amalgam and water, are reduced to symmetrical diols known as pinacol.
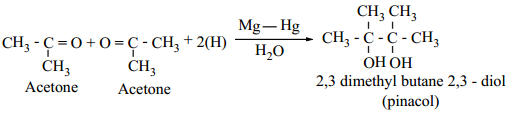
D. Haloform Reaction
Acetaldehyde and methyl ketones, containing image 26 group, when treated with halogen and alkali give the corresponding haloform. This is known as Haloform reaction.
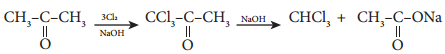
E. Reaction Involving Alkylgroup
(i) Aldol Condensation
The carbon attached to carbonyl carbon is called α – carbon and the hydrogen atom attached to α – carbon is called α – hydrogen.
In presence of dilute base NaOH, or KOH, two molecules of an aldehyde or ketone having α – hydrogen add together to give β – hydroxyl aldehyde (aldol) or β – hydroxyl ketone (ketol). The reaction is called aldol condensation reaction. The aldol or ketol readily loses water to give α, β – unsaturated compounds which are aldol condensation products.
(a) Acetaldehyde when warmed with dil NaOH gives β – hydroxyl butyraldehyde (acetaldol)
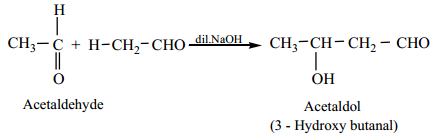
Mechanism
The mechanism of aldol condensation of acetaldehyde takes place in three steps.
Step 1:
The carbanion is formed as the α – hydrogen atom is removed as a proton by the base.
![]()
Step 2:
The carbanion attacks the carbonyl carbon of another unionized aldehyde to form an alkoxide ion.
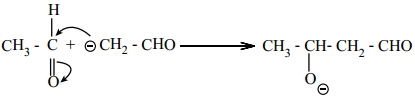
Step 3:
The alkoxide ion formed is protonated by water to form aldol.
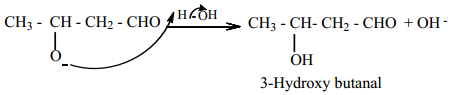
The aldol rapidly undergoes dehydration on heating with acid to form α – β unsaturated aldehyde.
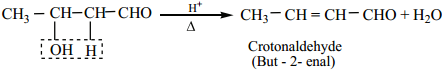
(ii) Crossed Aldol Condensation
Aldol condensation can also take place between two different aldehydes or ketones or between one aldehyde and one ketone such an aldol condensation is called crossed or mixed aldol condensation. This reaction is not very useful as the product is usually a mixture of all possible condensation products and cannot be separated easily.
Example
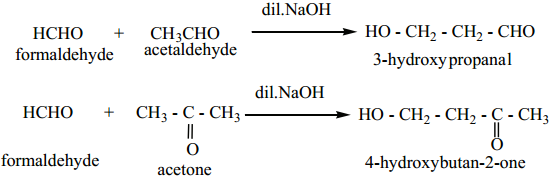
F. Some Important Reactions of Benzaldehyde
(i) Claisen – Schmidt Condensation
Benzaldehye condenses with aliphatic aldehyde or methyl ketone in the presence of dil. alkali at room temperature to form unsaturated aldehyde or ketone. This type of reaction is called Claisen – Schmidt condensation.
Example
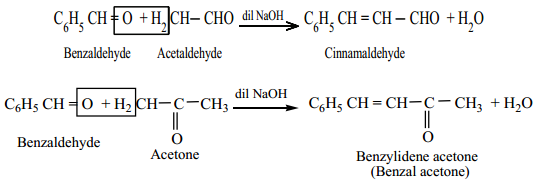
(ii) Cannizaro Reaction
In the presence of concentrated aqueous or alcoholic alkali, aldehydes which do not have α – hydrogen atom undergo self oxidation and reduction (disproportionation) to give a mixture of alcohol and a salt of carboxylic acid. This reaction is called Cannizaro reaction.
Benzaldehyde on treatment with concentrated NaOH (50%) gives benzyl alcohol and sodium benzoate.
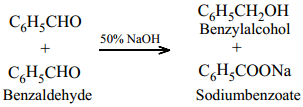
This reaction is an example disproportionation reaction
Mechanism of Cannizaro Reaction
Cannizaro reaction involves three steps.
Step 1:
Attack of OH– on the carbonyl carbon.
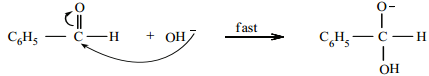
Step 2:
Hydride ion transfer
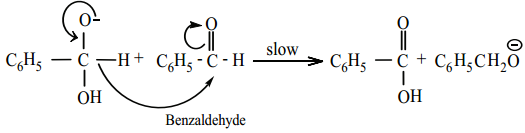
Step 3:
Acid – base reaction.
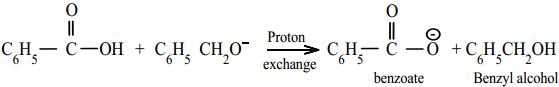
Cannizaro reaction is a characteristic of aldehyde having no α – hydrogen.
Crossed Cannizaro Reaction
When Cannizaro reaction takes place between two different aldehydes (neither containing an α hydrogen atom), the reaction is called as crossed cannizaro reaction.
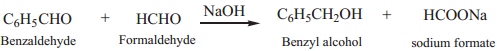
In crossed cannizaro reaction more reactive aldehyde is oxidized and less reactive aldehyde is reduced.
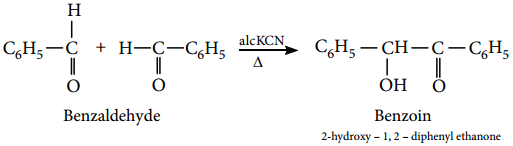
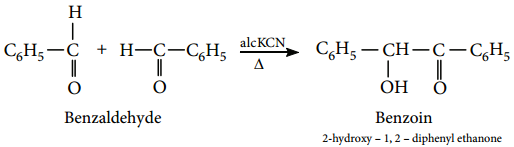
3. Benzoin Condensation
The Benzoin condensation involves the treatment of an aromatic aldehyde with aqueous alcoholic KCN. The products are a hydroxy ketone.
Example
Benzaldehyde reacts with alcoholic KCN to form benzoin
4. Perkins’ Reaction
When an aromatic aldehyde is heated with an aliphatic acid anhydride in the presence of the sodium salt of the acid corresponding to the anhydride, condensation takes place and an α, β unsaturated acid is obtained. This reaction is known as Perkin’s reaction.
Example
5. Knoevenagal Reaction
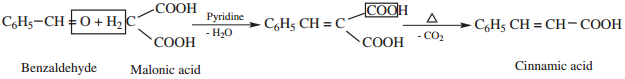
Benzaldehyde condenses with malonic acid in presence of pyridine forming cinnamic acid, Pyridine act as the basic catalyst.
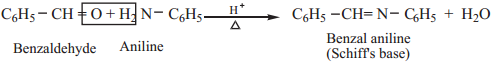
6. Reaction with Amine
Aromatic aldehydes react with primary amines (aliphatic or aromatic) in the presence of an acid to form schiff’s base.
Example
7. Condensation with Tertiary Aromatic Amines
Benzaldehyde condenses with tertiary aromatic amines like N, N – dimethyl aniline in the presence of strong acids to form triphenyl methane dye.
Example
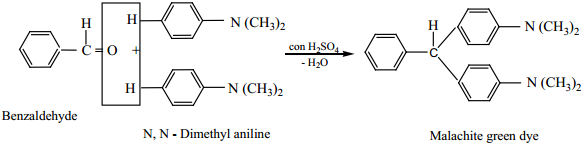
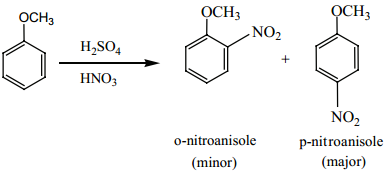
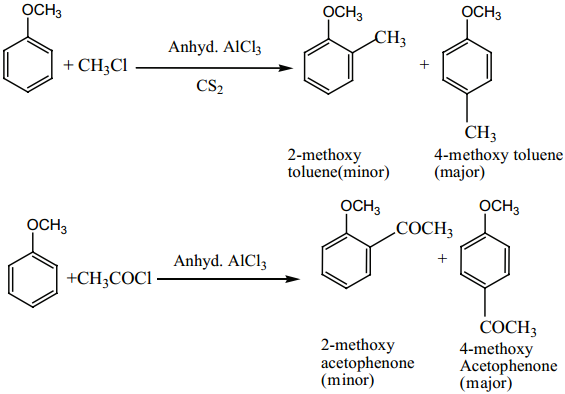
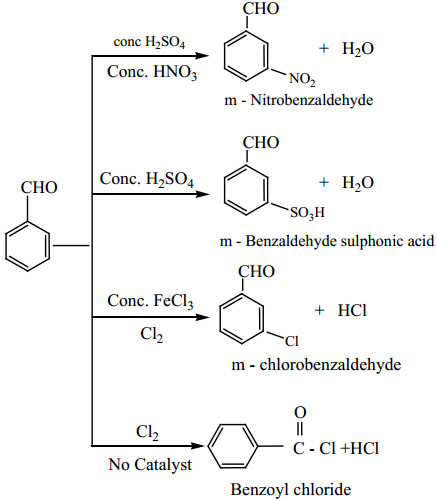
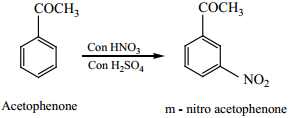
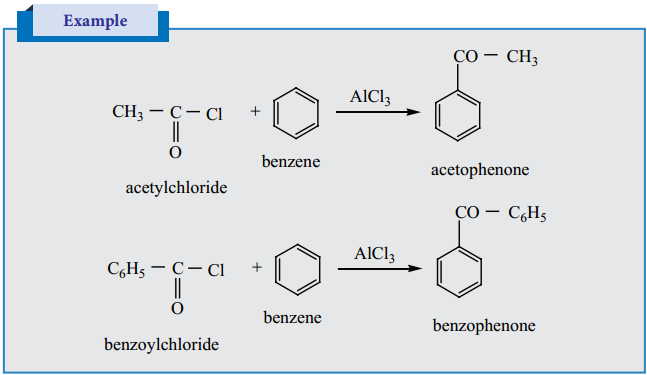
8. Electrophilic Substitution Reactions of Benzaldehyde
Electrophilic Substitution Reaction of Acetophenone
Acetophenone reacts with Nitrating mixture to form m – nitroacetophenone.
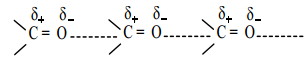
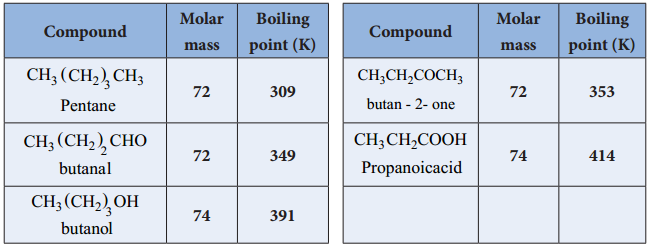
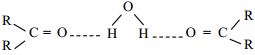
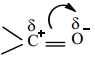
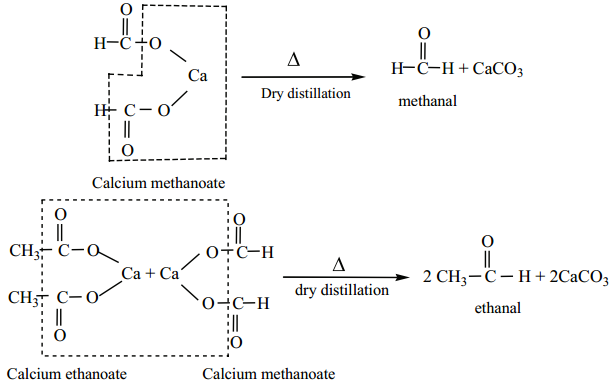
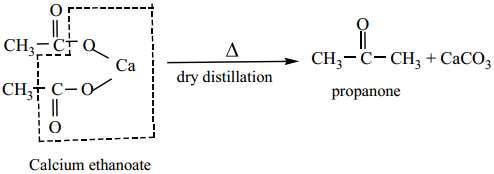
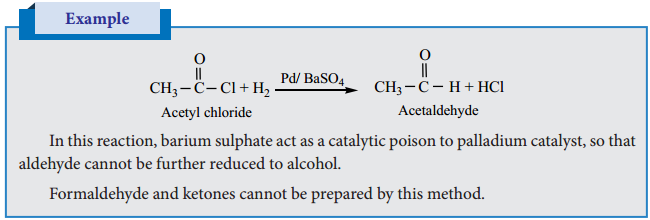
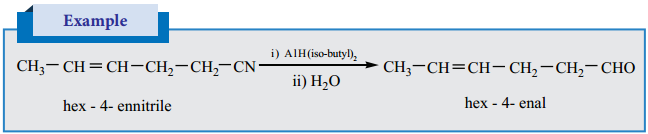
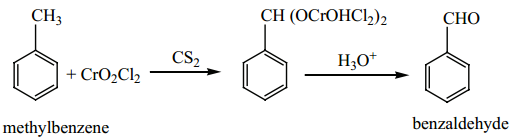
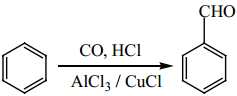
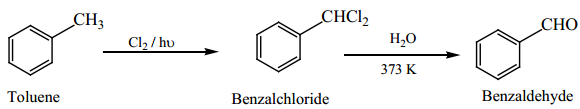
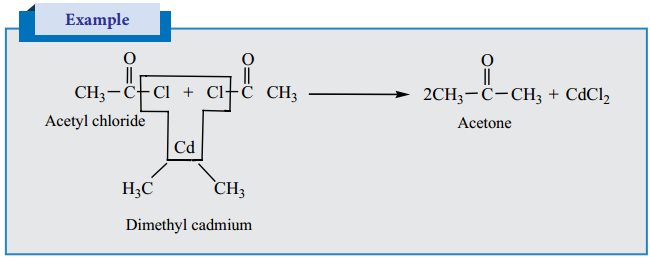
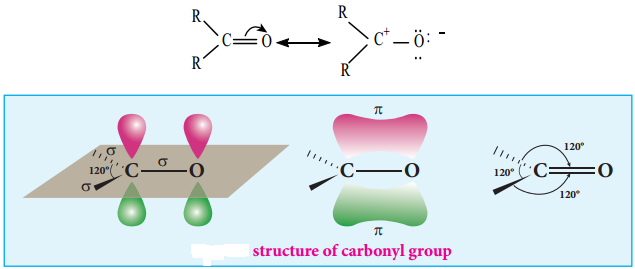
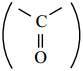 is sp2 hybridised and the carbon – oxygen bond is similar to carbon – carbon double bond in alkenes. The carbonyl carbon forms three σ bonds using their three sp2 hybridised orbital. One of the sigma bond is formed with oxygen and the other two with hydrogen and carbon (in aldehydes) or with two carbons (in ketones).
is sp2 hybridised and the carbon – oxygen bond is similar to carbon – carbon double bond in alkenes. The carbonyl carbon forms three σ bonds using their three sp2 hybridised orbital. One of the sigma bond is formed with oxygen and the other two with hydrogen and carbon (in aldehydes) or with two carbons (in ketones).