Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Physical Properties of Aldehydes and Ketones
1. Physical State:
Formaldehyde is a gas at room temperature and acetaldehyde is a volatile liquid. All other aldehydes and ketones upto to C11 are colourless liquids while the higher ones are solids.
2. Boiling Points
Aldehydes and ketones have relatively high boiling point as compared to hydrocarbons and ethers of comparable molecular mass. It is due to the weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
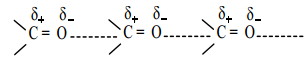
These dipole-dipole interactions are weaker than intermolecular H-bonding. The boiling points of aldehydes and ketones are much lower than those of corresponding alcohols and carboxylic acids which possess inter molecular hydrogen bonding.
3. Solubility
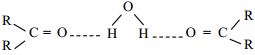
Lower members of aldehydes and ketones like formaldehyde, acetaldehyde and acetone are miscible with water in all proportions because they form hydrogen bond with water. Solubility of aldehydes and ketones decreases rapidly on increasing the length of alkyl chain.
4. Dipolemoment
The carbonyl group of aldehydes and ketones contains a double bond between carbon and oxygen. Oxygen is more electronegative than carbon and it attracts the shared pair of electron which makes the carbonyl group as polar and hence aldehydes and ketones have high dipole moments.
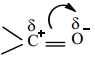
Solubility:
Aldehydes and ketones are soluble in water but their solubility decreases with increase in the length of the chain. Methanal, ethanal and propanone are those aldehydes and ketones which are of small size and are miscible with water in almost all proportions.
Physical Properties of Ketones
Ketones are highly reactive, although less so than aldehydes, to which they are closely related. Much of their chemical activity results from the nature of the carbonyl group. Ketones readily undergo a wide variety of chemical reactions.
Properties of Aldehydes
The reactivity of these compounds arises largely through two features of their structures:
The polarity of the carbonyl group and the acidity of any α-hydrogens that are present. Aldehydes are polar molecules, and many reagents seek atoms with a deficiency of electrons.
Both aldehydes and ketones contain a carbonyl group. That means that their reactions are very similar in this respect. An aldehyde differs from a ketone by having a hydrogen atom attached to the carbonyl group. This makes the aldehydes very easy to oxidise.
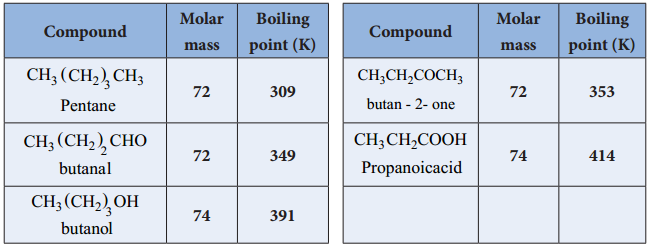
Carboxylic acids have high boiling points compared to other substances of comparable molar mass. Boiling points increase with molar mass. Carboxylic acids having one to four carbon atoms are completely miscible with water. Solubility decreases with molar mass.
Aldehydes and ketones have a much higher boiling point than the alkanes. As the molecules get larger, the difference between an aldehyde/ketone and its corresponding alkane gets smaller. The reason for this is that the non-polar region of the carbon chain is getting larger as the polar region (C=O) is staying the same.
Examples of Aldehydes
Aldehydes are given the same name but with the suffix – ic acid replaced by – aldehyde. Two examples are formaldehyde and benzaldehyde. As another example, the common name of CH2=CHCHO, for which the IUPAC name is 2-propenal, is acrolein, a name derived from that of acrylic acid, the parent carboxylic acid.