Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Cyanides and Isocyanides
These are the derivatives of hydrocyanic acid (HCN), and is known to exist in two tautomeric forms

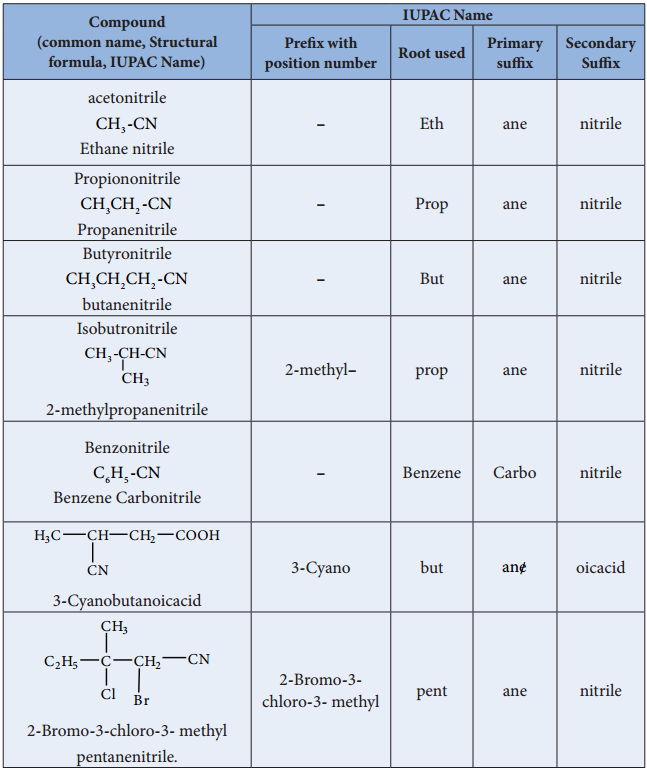
Two types of alkyl derivatives can be obtained. Those derived by replacement of H – atom of hydrogen cyanide by the alkyl groups are known as alkyl cyanides (R-C≡N) and those obtained by the replacement of H – atom of hydrogen isocyanide are known as alkyl isocyanides
![]()
In IUPAC system, alkyl cyanides are named as “alkanenitriles” whereas aryl cyanides as “arenecarbonitrile”.
Table: Nomenclature of Cyanides
Methods of Preparation of Cyanides
1. From Alkyl Halides
When alkyl halides are treated in the solution NaCN (or) KCN, alkyl cyanides are obtained. In this reaction a new carbon – carbon bond is formed.
Example
![]()
Aryl cyanide cannot be prepared in this method because of their less reactivity towards nucleophilic substitution. Aryl cyanides are prepared using Sandmeyers reactions.
2. By Dehydration of Primary Amides and Aldoximes with P2O5

3. By dehydration of ammonium carboxylates with P2O5
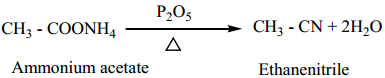
This method suitable for large scale preparation of alkyl cyanides.
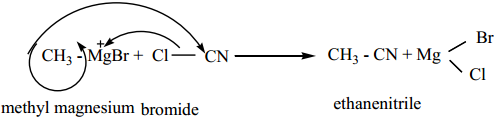
4. From Grignard Reagent
Methyl magnesium bromide on treatment with cyanogen chloride (Cl – CN) forms ethanenitrile.
Properties of Cyanides
Physical Properties
The lower members (up to C14) are colourless liquids with a strong characteristic sweet smell. The higher members are crystalline solids, They are moderately soluble in water but freely souble in organic solvents. They are poisonous. They have higher boiling points than analogous acetylenes due to their high dipole moments.
Chemical Properties
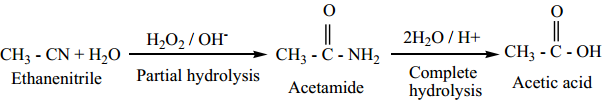
1. Hydrolysis
On boiling with alkali (or) a dilute mineral acid, the cyanides are hydrolysed to give carboxylic acids.
For Example
2. Reduction
On reduction with LiAlH4 2(or) Ni/H2, alkyl cyanides yields primary amines.
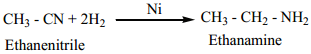
3. Condensation Reaction
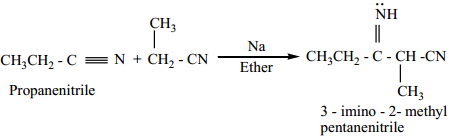
(a) Thorpe Nitrile Condensation
Self condensation of two molecules of alkyl nitrile (containing α-H atom) in the presence of sodium to form iminonitrile.
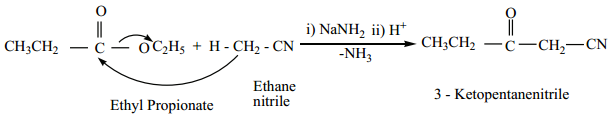
(b) The nitriles containing α – hydrogen also undergo condensation with esters in the presence of sodamide in ether to form ketonitriles. This reaction is known as “Levine and Hauser” acetylation.
This reaction involves replacement of ethoxy (OC2H5) group by methylnitrile (- CH2CN) group and
is called as cyanomethylation reaction.
Alkyl Isocyanides (Carbylamines)
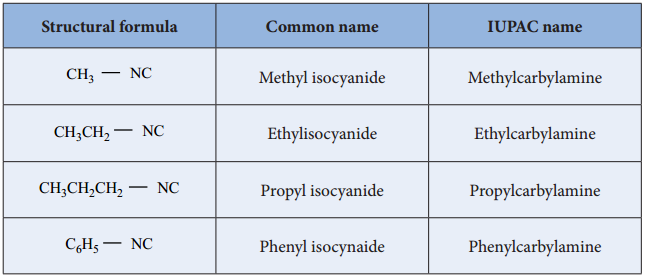
Nomenclature of Isocyanides
They are commonly named as Alkyl isocyanides. The IUPAC system names them as alkylcarbylamines
Table: Nomenclature of Alkylisocyanides
Methods of Preparation of Isocyanides
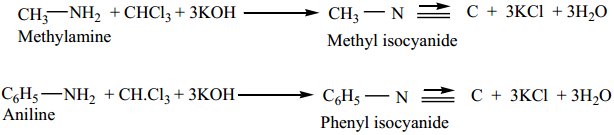
1. From Primary Amines (Carbylamines Reaction)
Both aromatic as well as aliphatic amines on treatment with CHCl3 in the presence of KOH give carbylamines.
2. From Alkyl Halides
Ethyl bromide on heating with ethanolic solution of AgCN give ethyl isocyanide as major product and ethyl cyanide as minor product.
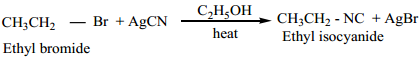
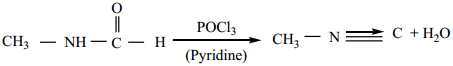
3. From N – alkyl formamide. By reaction with POCl3 in pyridine.
Properties of Isocyanides
Physical Properties
- They are colourless, highly unpleasant smelling volatile liquids and are much more poisonous than the cyanides.
- They are only slightly soluble in water but are soluble in organic solvents.
- They are relatively less polar than alkyl cyanides. Ths, their melting point and boiling point are lower than cyanides.
Chemical Properties
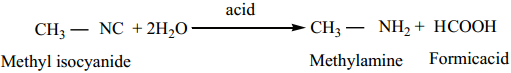
1. Hydrolysis:
Alkyl isocyanides are not hydrolysed by alkalies. However they are hydrolysed with dilute mineral acids to give primary amines and formic acids.
2. Reduction:
When reduced catalytically (or) by nascent hydrogen, they give secondary amines.
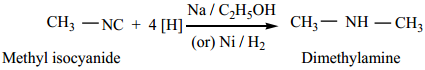
3. Isomerisation:
When Alkyl isocyanides and heated at 250°C, they change into the more stable, isomeric cyanides

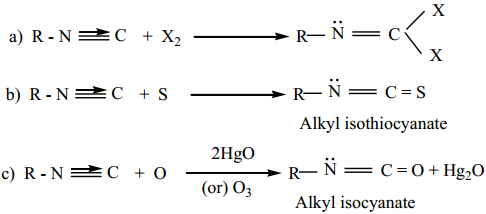
4. Addition Reaction:
Alkyl isocyanides add on halogen, sulphur, and oxygen to form the corresponding addition compounds.
Uses of Organic Nitrogen Compounds
Nitroalkanes
- Nitromethane is used as a fuel for cars
- Chloropicrin (CCl3NO2) is used as an insecticide
- Nitroethane is used as a fuel additive and precursor to explosive and they are good solvents for polymers, cellulose ester, synthetic rubber and dyes etc.,
- 4% solution of ethylnitrite in alcohol is known as sweet spirit of nitre and is used as diuretic.
Nitrobenzene
- Nitrobenzene is used to produce lubricating oils in motors and machinery.
- It is used in the manufacture of dyes, drugs, pesticides, synthetic rubber, aniline and explosives like TNT, TNB.
Cyanides and Isocyanides
1. Alkyl cyanides are important intermediates in the organic synthesis of larger number of compounds like acids, amides, esters, amines etc.
2. Nitriles are used in textile industry in the manufacture of nitrile rubber and also as a solvent particularly in perfume industry.