Corrosion of Metals:
The phenomenon of a surface of metal being attacked by air, water or any other substance around it is known as corrosion and the metal being attacked is said to corrode.
Examples of corrosion:
- When iron is exposed to moist air for a long time, its surface acquires a coating of a brown, flaky substance called rust, which is mainly hydrated iron oxide (Fe203.xH20).
- The surface of copper acquires a green coating of basic copper carbonate in moist air.
- The surface of aluminium gets coated with a thin layer of aluminium oxide which prevents the metal underneath from further damage.
- Silver articles become black after sometime when exposed to air as silver reacts with sulphur present in the air to form a coating of siLver sulphide.
- Noble metals such as goLd and silver do not corrode readily. Both air and water are necessary for corrosion to take place.
![]()
Example 1.
You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer:
Copper vessels become tarnished when the copper metal gets corroded and forms a layer of basic copper carbonate by reacting with carbon dioxide present in air.
Sour substances such as lemonjuice ortamarind juice are acidic in nature as they contain citric acid and tartaric acid respectively. Being acidic, they neutralize the basic copper carbonate and dissolves the layer of copper carbonate formed.
Prevention of Corrosion
Rusting of iron can be prevented by painting, oiling and greasing, galvanizing (by coating iron objects with zinc), chrome plating etc.
Galvanization:
Galvanisation is the method of protecting steel and iron from rusting by coating them with a thin layer of zinc. The galvanized article remains protected from corrosion even if the zinc layer is broken.
Important
If the zinc coating is broken, the galvanised object remains protected against rusting because zinc is more reactive than iron and hence can be easily oxidised .Thus when zinc layer breaks down, the zinc continues to react and gets oxidized.
Alloying:
Alloying is a method for improving the properties of a metal thereby getting desired properties.
Example: Pure iron is very soft and stretches easily when hot. It is therefore never used in its pure state, but instead mixed with a small amount of carbon (0.05 %) due to which it becomes hard and strong.
Alloys: An alloy is a homogeneous mixture of two or more metals, or a metal and a non-metal. It is prepared by first melting the main metal and then dissolving the other elements in it in a definite proportion.
![]()
Amalgam:
An alloy whose one of the metals is mercury is known as an amalgam.
Properties of alloys:
- The electrical conductivity of an alloy is less than that of pure metals.
- Sometimes an alloy has lower melting point than any of its constituents.
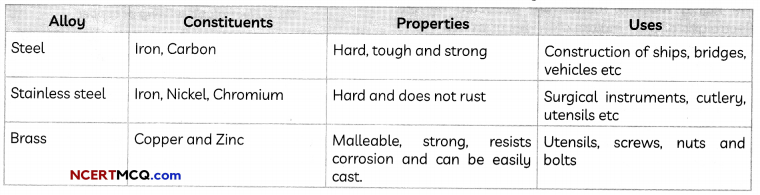
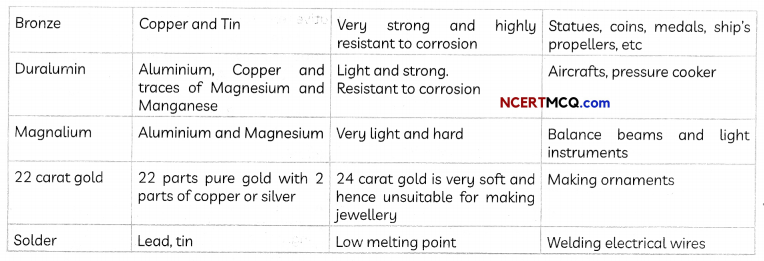
Some common alloys: