Covalent Bonding in Carbon:
The amount of carbon present in the earth’s crust and in the atmosphere is very less. The earth’s crust has only 0.02% carbon in the form of minerals (like carbonates, hydrogencarbonates,coal and petroleum) and the atmosphere has 0.03% of carbondioxide. Yet, we find that a large number of things that we use in our daily life are made of carbon compounds. Food, clothes, medicines, books and many other things are some examples.
The presence of carbon in a material can be tested by burning the substance in air and passing the gas formed through lime water. If the lime water turns milky, then the given material contains carbon.
Most carbon compounds are poor conductors of electricity and have low boiling and melting points, from which it can be concluded that the forces of attraction between these molecules are not very strong. Since these compounds are largely non-conductors of electricity, we can conclude that the bonding in these compounds does not give rise to any ions.
In the case of carbon, it has four electrons in its outermost shell and in order to attain noble gas
configuration, it needs to either gain four electrons or lose four electrons.
- It could gain four electrons forming C4- anion. But as the nucleus contains only six protons, it would be difficult for the nucleus to hold on to ten electrons.
- It could lose four electrons forming a C4+ cation. But a large amount of energy would be required to remove four electrons leaving behind a carbon cation with six protons in its nucleus holding on to just two electrons.
Carbon overcomes this problem by sharing its valence electrons with other atoms of carbon or with atoms of other elements. Apart from carbon, there are many other elements also which form molecules by sharing electrons in this manner. The shared electrons ‘belong’ to the outer shells of both the atoms and lead to both atoms attaining the noble gas configuration.
Covalent Bond: The types of bonds that are formed by the sharing of an electron pair between two atoms are known as covalent bonds.
Depending upon the number of pairs of electrons shared between atoms, there can be single, double or triple covalent bonds.
![]()
Some Examples of Covalent Compounds

Example 1.
Draw the electron dot structures for
(A) Ethanoic acid.
(B) H2S
(C) Propanone.
(D) F2
Answer:
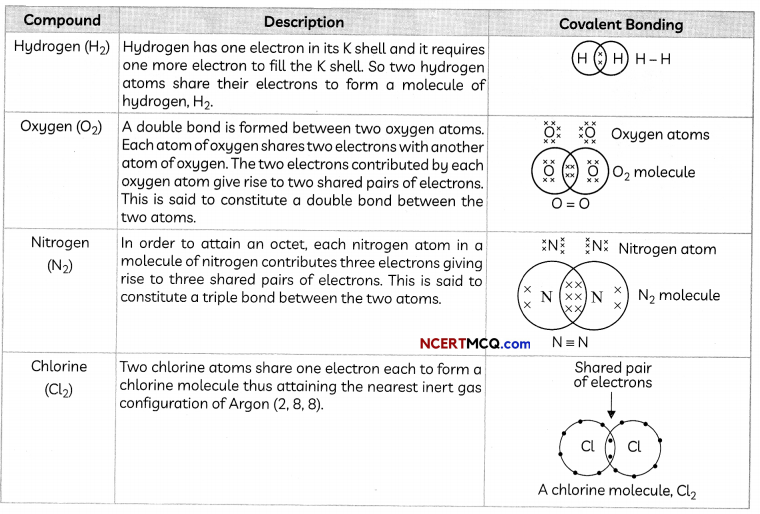
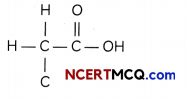
(A) Ethanoic acid (CH3COOH):
A number of valence electrons of carbon = 4.
hydrogen = 1. oxygen = 6
Structural formula and electron dot structure is given below:
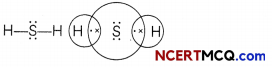
(B) Hydrogen suLphide (H2S):
Number of valence electrons of sulphur = 6, hydrogen = 1
Structural formula and electron dot structure is given:
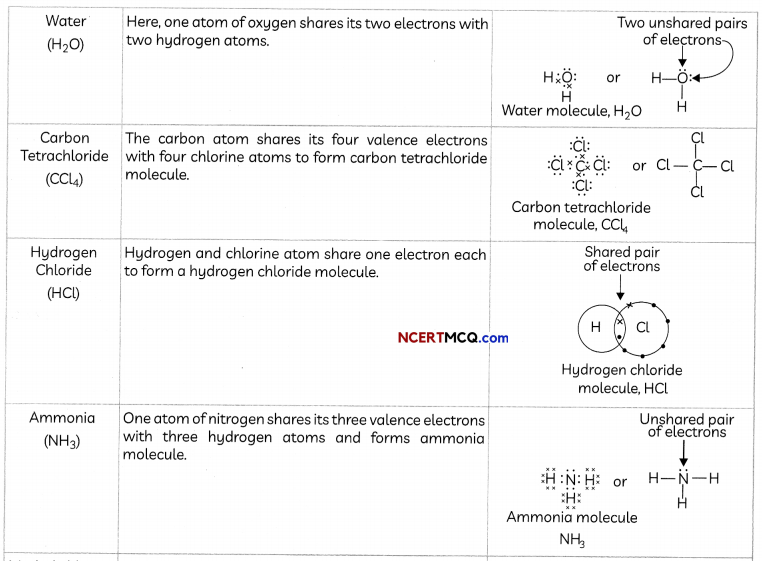
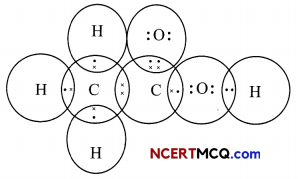
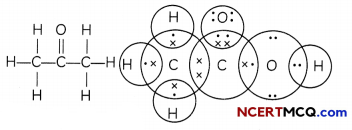
(C) Propanone (CH3COCH3):
Number of valence electrons of carbon = 4,
hydrogen = 1, oxygen = 6
Structural formula and electron dot structure is given as:
(D) FLuorine (F2):
Number of valence electrons of fluorine = 7
The structural formula and electron dot structure is given below:

![]()
Properties of Covalent Compounds
| Property | Description |
| 1. Covalent compounds are usually liquids or gases | 1. This is due to the weak forces of attraction between their molecules. |
| 2. Covalent compounds have usually low melting and boiling points | 2. Covalently bonded molecules are seen to have strong bonds within the molecule, but intermolecular forces are small |
| 3. Usually insoluble in water but soluble in organic solvents | 3. This is also due to the presence of strong bonds within the molecule and small intermolecular forces |
| 4. Covalent compounds do not conduct electricity | 4. Since the electrons are shared between atoms and no charged particles are formed, such covalent compounds are generally poor conductors of electricity. |
Differences between Ionic Bond and Covalent Bond
| Ionic Bond | Covalent Bond |
| 1. An ionic bond is a chemical bond between two dissimilar (i.e. a metal and a non-metal) atoms in which one atom gives up an electron to another. | 1. In a covalent bond the two atoms come together to share the electron, instead of an atom taking an electron from another |
| 2. An ionic bond is formed between a metal and a non-metal. | 2. A covalent bond is formed between two non-metals that have similar electronegativities. |
| 3. Molecules have no definite shapes, as they have lattice structures | 3. Molecules have a definite shape. |
| 4. Electrical and thermal conductivity is High | 4. No electrical conductivity but Thermal conductivity is usually low |
| 5. Usually High melting point | 5. Lower melting point |
| 6.Usually highly soluble in water | 6. lower solubility |
| 7. Usually solids at room temperature | 7. Exists as solids, liquids, gases |
Allotropes of Carbon:
The various physical forms in which an element can exist are called allotropes of the element. Carbon exists in three solid forms called allotropes. The three alLotropes of carbon are:
- Diamond
- Graphite
- Fullerenes
![]()
Diamond
- Diamonds are colourless, transparent, sparkle and reflect light, which is why they are described as Lustrous.
- It is extremely hard and has a high melting point.
- It does not conduct electricity.
Structure of diamond: Diamond is one giant molecule of carbon atoms. Every atom in a diamond is bonded to its neighbours by four strong covalent bonds, leaving no free electrons and no ions. This explains why diamond does not conduct electricity.
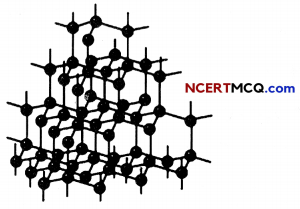
The Structure of Diamond
Uses of diamond:
- Diamond is used in cutting instruments like glass cutters and in rock drilling equipment, as it is extremely hard.
- Diamonds are used for making jewellery.
- Sharp-edged diamonds are used by eye surgeons as a tool to remove cataract.
Graphite
- Graphite is black, shiny and opaque.
- It is a very slippery material.
- Graphite is insoluble in water.
- It has a high melting point and is a good conductor of electricity, which makes it a suitable material for the electrodes needed in electrolysis.
Structure of graphite: Graphite contains layers of carbon atoms.ln graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonaL array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other.
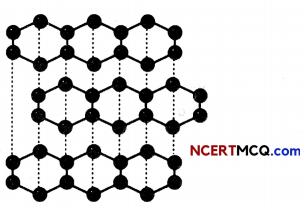
The Structure of Graphite
![]()
Uses of graphite:
- Powdered graphite is used as a lubricant for the fast-moving parts of machinery.
- It is used for making electrodes in dry cells and electric arcs as it is a very good conductor of electricity.
- It is used for making the core of pencils called ‘pencil Leads’.
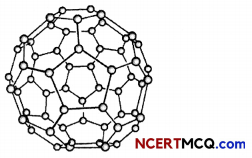
Fullerenes:
Fullerenes form another class of carbon allotropes. The first one to be identified was C-60 which has carbon atoms arranged in the shape of a football. Since this looked like the geodesic dome designed by the US architect Buckminster Fuller, the molecule was named fullerene.
Structure of C-60 Bacminster Fullerene