Early Attempts at Classification of Elements
The number of elements known to us is around 118. But around the year 1800, only 30 elements were known and their properties were also not known much. With the discovery of more elements, scientists gathered more and more information about the properties of these elements and looked for ways to organize them on the basis of similarities in their properties.
The earliest attempt to classify the elements resulted in grouping the then-known elements as metals and non-metals. Later further classifications were tried out as our knowledge of elements and their properties increased.
Dobereiner’s Triads:
In the year 1817, Johann Wolfgang Dobereiner, a German chemist, identified some groups having three elements each of which he called ‘triads’.
Dobereiner showed that when the three elements in a triad were written in the order of increasing atomic masses, the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
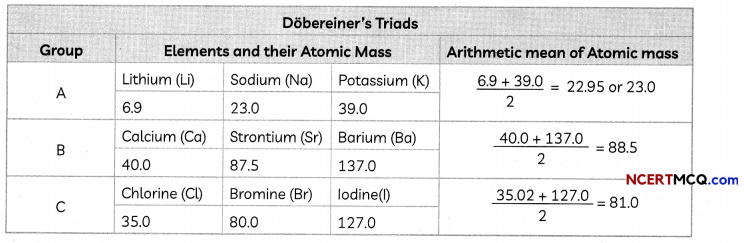
Example: Consider the triad consisting of lithium (Li), sodium (Na), and potassium (K) with the respective atomic masses 6.9, 23.0, and 39.0. The atomic mass of sodium (23) is the mean of the masses of lithium and potassium.
Achievements of Dobereiner’s Triads:
This was a great step in predicting the atomic mass and properties of middle elements. The triads identified by Dobereiner are placed in the same group even in the Modern Periodic Table.
![]()
Example 1.
What were the limitations of Dobereiner’s classification?
Answer:
All the elements discovered at that time could not be grouped into triads as Dobereiner could identify only three triads from amongst the elements known at that time.
Take the example of N, P and As. Atomic mass of P (31.0) is not an arithmetic mean of atomic masses of N (14.0) and As (74.9), which is 44.4.
Newlands’ Law of Octaves
In 1866, John Newlands, an English scientist, arranged the then-known elements in the order of increasing atomic masses. He started with the element having the lowest atomic mass (hydrogen) and ended at thorium which was the 56th element.
He found that every eighth element had properties similar to that of the first. He compared this to the octaves found in music. Therefore, he called it the ‘Law of Octaves’. It is known as ‘Newlands’ Law of Octaves’. In Newlands’ Octaves, the properties of lithium and sodium were found to be the same. Sodium is the eighth element after lithium. Similarly, beryllium and magnesium resemble each other.
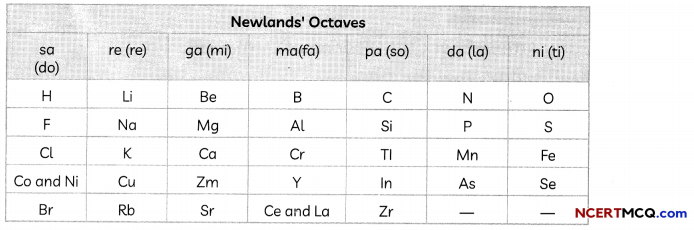
Limitations of Newland’s Law of Octaves
1. The Law of Octaves was applicable only up to calcium, as after calcium every eighth element did not possess properties similar to that of the first.
2. Newlands assumed that only 56 elements existed in nature and no more elements would be discovered in the future. But, later on, several new elements were discovered, whose properties did not fit into the Law of Octaves.
3. In order to fit elements into his Table, Newlands adjusted two elements in the same sLot, but also put some unlike elements under the same note. Example:
- Cobalt and nickel are in the same slot and these are placed in the same column as fluorine, chlorine, and bromine which have very different properties than these elements.
- Iron, which resembles cobalt and nickel in properties, has been placed far away from these elements.
4. Newlands’ Law of Octaves worked well with lighter elements only.
![]()
Example 2.
Did Dobereiner’s triads also exist in the columns of Newlands’ Octaves? Compare and find out.
Answer:
Yes, Dobereiner’s triads also exist in the columns of Newlands’ Octaves. For example, Li, Na and K form Dobereiner’s triads and they also exist in Newland’s octaves under the same note or column.