Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Extraction of Crude Metal
The extraction of crude metals from the concentrated ores is carried out in two steps namely
- Conversion of the ore into oxides of the metal of interest and
- Reduction of the metal oxides to elemental metals. In the concentrated ore, the metal exists in positive oxidation state and hence it is to be reduced to its elemental state.
- We can infer from the principles of thermodynamics, that the reduction of oxide is easier when compared to reduction of other compounds of metal and hence, before reduction, the ore is first converted into the oxide of metal of interest.
- Let us discuss some of the common methods used to convert the concentrated ore into the oxides of the metal of interest.
Conversion of Ores into Oxides
Roasting
Roasting is the method, usually applied for the conversion of sulphide ores into their oxides. In this method, the concentrated ore is oxidised by heating it with excess of oxygen in a suitable furnace below the melting point of the metal.
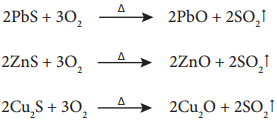
Roasting also removes impurities such as arsenic, sulphur, phosphorous by converting them into their volatile oxides.
For Example
4As + 3O2 → 2As2O3 ↑
S8 + 😯2 → 8SO2 ↑
P4 + 5O2 → P4O10 ↑
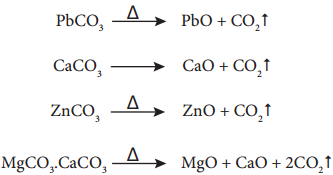
Calcination
Calcination is the process in which the concentrated ore is strongly heated in the absence of air. During this process, the water of crystallisation present in the hydrated oxide escapes as moisture. Any organic matter (if present) also get expelled leaving behind a porous ore. This method can also be carried out with a limited supply of air.
For examples,
During calcination of carbonate ore, carbon dioxide is expelled
During calcination of hydrated ore, the water of hydration is expelled as vapour
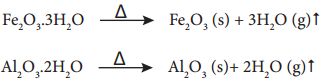
Reduction of Metal Oxides
Metal oxide can be reduced to crude metal by using a suitable reducing agent like carbon, carbon monoxide, hydrogen, aluminium and other reactive metals such as sodium etc. The choice of reducing agent depends on the nature of the metal.
For example, carbon cannot be used as a reducing agent for the reactive metals such as sodium, potassium, aluminium etc. Similarly CO cannot be used to reduce oxides such as ZnO, Al2O3. Later in this, we study selection of suitable reducing agents by applying ellingham diagram.
Smelting
In this method, a flux (a chemical substance that forms an easily fusible slag with gangue) and a reducing agent such as carbon, carbon monoxide (or) aluminium is added to the concentrated ore and the mixture is melted by heating at an elevated temperature (above the melting point of the metal) in a smelting furnace. For example the oxide of iron can be reduced by carbon monoxide as follows.
Fe2O3(s) + 3CO (g) → 2Fe(s) + 3CO2(g)↑
In this extraction, a basic flux, limestone (CaO) is used. Since the silica gangue present in the ore is acidic in nature, the limestone combines with it to form calcium silicate (slag).
![]()
In the extraction of copper from copper pyrites, the concentrated ore is heated in a reverberatory furnace after mixing with silica, an acidic flux. The ferrous oxide formed due to melting is basic in nature and it combines with silica to form ferrous silicate (slag). The remaining metal sulphides Cu2S and FeS are mutually soluble and form a copper matte.
2CuFeS2(s) + O2(g) → 2Cu2(l, s) + 2SO2 (g)
2Cu2O(l) + Cu2S (l) → 6Cu(l) + SO2(g)
The matte is separated from the slag and fed to the converting furnace. During conversion, the FeS present in the matte is first oxidised to FeO. This is removed by slag formation with silica. The remaining copper sulphide is further oxidised to its oxide which is subsequently converted to metallic copper as shown below.
2Cu2S (l, s) + 3O2 (g) → 2Cu2O (l, s) + 2SO2(g)
2Cu2O(l) + Cu2S (l) → 6Cu (l) + SO2 (g)
The metallic copper is solidified and it has blistered appearance due to evolution of SO2 gas formed in this process. This copper is called blistered copper.
Reduction By Carbon:
In this method the oxide ore of the metal is mixed with coal (coke) and heated strongly in a furnace (usually in a blast furnace). This process can be applied to the metals which do not form carbides with carbon at the reduction temperature.
Examples:
ZnO (s) + C (s) → Zn (s) + CO (g)↑
Mn3O4 (s) + 4C (s) → 3Mn (s) + 4CO (g)↑
Cr2O3 (s) + 3C (s) → 2Cr (s) + 3CO (g)↑
Reduction By Hydrogen:
This method can be applied to the oxides of the metals (Fe, Pb, Cu) having less electropositive character than hydrogen.
Ag2O (s) + H2 (g) → 2Ag (s) + H2O (l)
Fe3O4 (s) + 4H2 (g) → 3Fe (s) + 4H2O (l)
Nickel oxide can be reduced to nickel by using a mixture of hydrogen and carbon monoxide (water gas)
2NiO (s) + CO (g) + H2 (g) → 2Ni (s) + CO2 (g) + H2O (l)
Reduction By Metal:
Metallic oxides such as Cr2O3 can be reduced by an aluminothermic process. In this process, the metal oxide is mixed with aluminium powder and placed in a fire clay crucible. To initiate the reduction process, an ignition mixture (usually magnesium and barium peroxide) is used.
BaO2 + Mg → BaO + MgO
During the above reaction a large amount of heat is evolved (temperature up to 2400°C, is generated and the reaction enthalpy is : 852 kJ mol-1) which facilitates the reduction of Cr2O3 by aluminium power.
Active metals such as sodium, potassium and calcium can also be used to reduce the metal oxide
B2O3 + 6Na → 2B + 3Na2O
Rb2O3 + 3Mg → 2Rb + 3MgO
TiO2 + 2Mg → Ti + 2MgO
![]()
Auto-Reduction:
Simple roasting of some of the ores give the crude metal. In such cases, the use of reducing agents is not necessary. For example, mercury is obtained by roasting of its ore cinnabar (HgS).
HgS (s) + O2 (g) → Hg (l) + SO2↑