Find free online Chemistry Topics covering a broad range of concepts from research institutes around the world.
Functional Derivatives of Carboxylic Acids
Compounds such as acid chlorides, amides, esters etc., are called carboxylic acid derivatives because they differ from a carboxylic acid only in the nature of the group or atom that has replaced the – OH group of carboxylic acid.
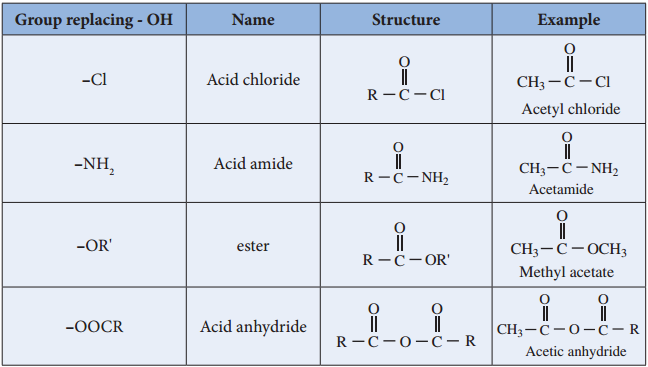
Relative Reactivity of Acid Derivatives
The reactivity of the acid derivatives follows the order
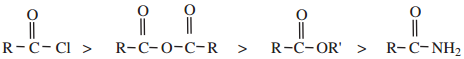
The above order of reactivity can be explained in terms of
- Basicity of the leaving group
- Resonance effect
1. Basicity of the Leaving Group
Weaker bases are good leaving groups. Hence acyl derivatives with weaker bases as leaving groups (L) can easily rupture the bond and are more reactive. The correct order of the basicity of the leaving group is ![]() . Hence the reverse is the order of reactivity.
. Hence the reverse is the order of reactivity.
2. Resonance Effect
Lesser the electronegativity of the group, greater would be the resonance stabilization as shown below. This effect makes the molecule more stable and reduces the reactivity of the acyl compound. The order of electronegativity of the leaving groups follows the order – Cl > – OCOR > – OR > – NH2
Hence the order of reactivity of the acid derivatives with nucleophilic reagent follows the order
acid halide > acid anhydride > esters > acid amides
Nomenclature
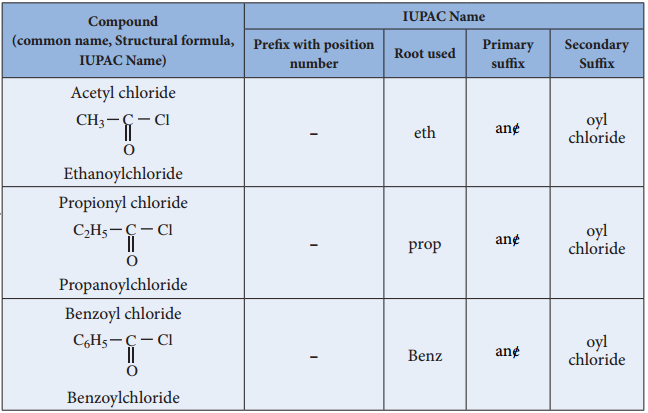
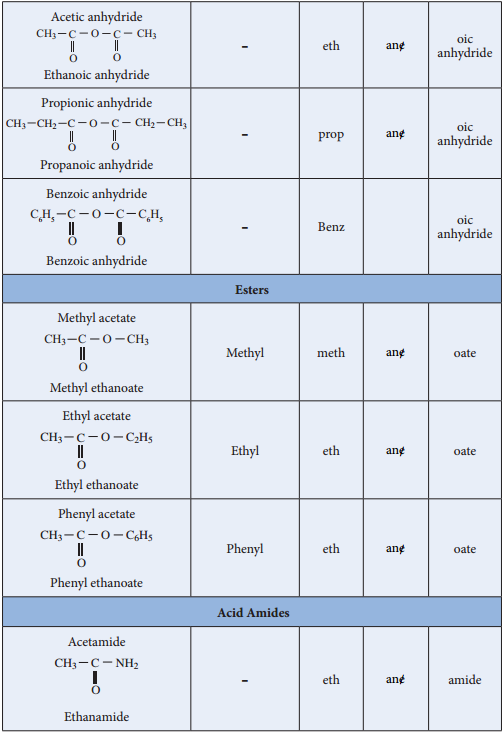
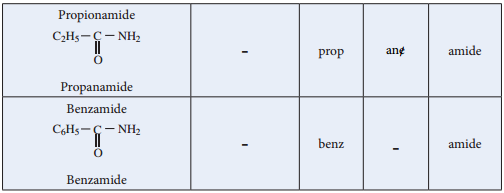
Acid Halides:
Methods of Preparation of Acid Chloride:
Acid chlorides are prepared from carboxylic acid by treating it with anyone of the chlorinating agent such as SOCl2, PCl5, or PCl3
1. By Reaction with Thionyl Chloride (SOCl2)
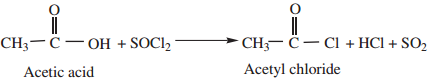
This method is superior to others as the by products being gases escape leaving the acid chloride in the pure state.
Physical Properties:
- They emit pale fumes of hydrogen chloride when exposed to air on account of their reaction with water vapour.
- They are insoluble in water but slowly begins to dissolve due to hydrolysis.
Chemical Properties:
They react with weak nucleophiles such as water, alcohols, ammonia and amines to produce the corresponding acid, ester, amide or substituted amides.
1. Hydrolysis:
Acyl halides undergo hydrolysis to form corresponding carboxylic acids
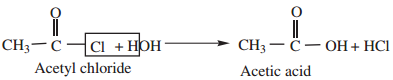
2. Reaction with Alcohols (Alcoholysis) gives esters.
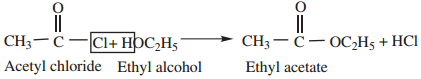
3. Reaction with Ammonia (Ammonolysis) gives acid amides.
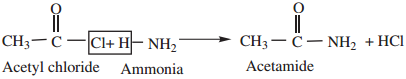
4. Reaction with 1° and 2° Amines gives N-alkyl amides.
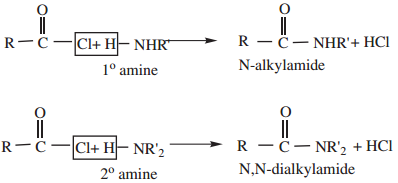
5. Reduction
(a) When reduced with hydrogen in the presence of ‘poisoned’ palladium catalyst, they form aldehydes. This reaction is called Rosenmund reduction. We have already learnt this reaction under the preparation of aldehydes.
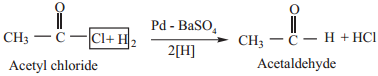
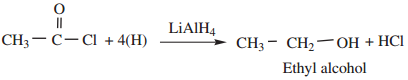
(b) When reduced with LiAlH4 gives primary alcohols.
Acid Anhydride
Methods of Preparation
1. Heating carboxylic acid with P2O5
We have already learnt that when carboxylic acids are heated with P2O5 dehydration takes place to form
acid anhydride.
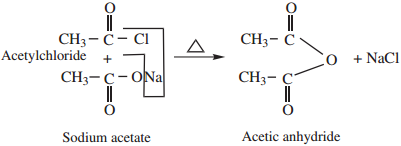
2. By Reaction of Acid Halide With a Salt of Carboxylic Acids
Acid chlorides on heating with sodium salt of carboxylic acids gives corresponding anhydride.
Chemical Properties
1. Hydrolysis
Acid anhydride are slowly hydrolysed, by water to form corresponding carboxylic acids.
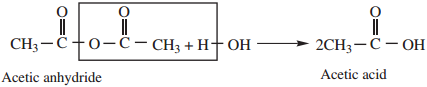
2. Reaction With Alcohol
Acid anhydride reacts with alcohols to form esters.
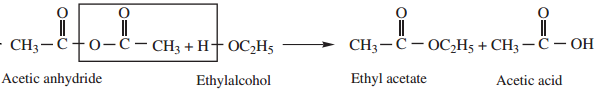
3. Reaction With Ammonia
Acid anhydride reacts with ammonia to form amides.
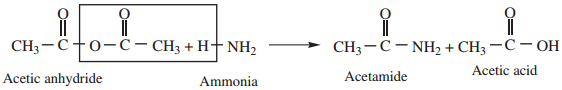
4. Reaction with PCl5
Acid anhydride reacts with PCl5 to form acyl chlorides.
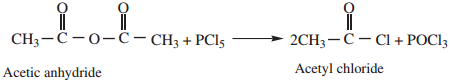
Esters
Methods of Preparation
1. Esterification
We have already learnt that treatment of alcohols with carboxylic acids in presence of mineral acid gives esters. The reaction is carried to completion by using an excess of reactant or by removing the water from the reaction mixture.
2. Alcoholysis of Acid Chloride or Acid Anhydrides
(ii) Treatment of acid chloride or acid anhydride with alcohol also gives esters.
Physical Properties
Esters are colour less liquids or solids with characteristic fruity smell. Flavours of some of the esters are given below.
|
Ester |
Flavour |
| 1. Amyl acetate | Banana |
| 2. Ethyl butyrate | Pineapple |
| 3. Octyl acetate | Orange |
| 4. Isobutyl formate | Raspberry |
| 5. Amyl butyrate | Apricot |
Chemical Properties
They react with weak nucleophiles such as water, alcohols, ammonia and amines to produce the corresponding acid, ester, amide or substituted amides.
1. Hydrolysis
We have already learnt that hydrolysis of esters gives alcohol and carboxylic acid.
2. Reaction With Alcohol (Transesterification)
Esters of an alcohol can react with another alcohol in the presence of a mineral acid to give the ester of second alcohol. The interchange of alcohol portions of the esters is termed transesterification.
Transesterification
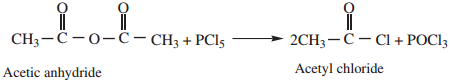
3. Reaction With Ammonia (Ammonolysis)
Esters react slowly with ammonia to form amides and alcohol.
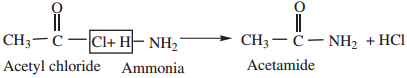
4. Claisen Condensation
Esters containing at least one ∝ – hydrogen atom undergo self condensation in the presence of a strong base such as sodium ethoxide to form β – keto ester.
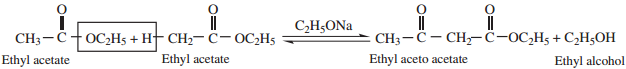
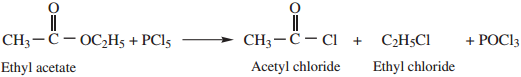
5. Reaction with PCl5
Esters react with PCl5 to give a mixture of acyl and alkyl chloride
Acid Amides
Acid amides are derivatives of carboxylic acid in which the – OH part of carboxyl group has been replaced by – NH2 group. The general formula of amides are given as follows image 21 Now, we shall focus our attention mainly on the study of chemistry of acetamide.
Methods of Preparation
1. Ammonolysis of Acid Derivatives
Acid amides are prepared by the action of ammonia with acid chlorides or acid anhydrides.
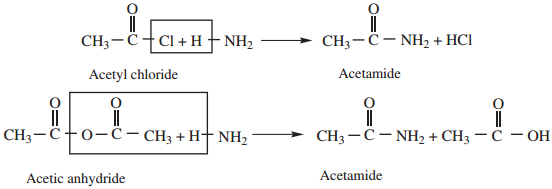
2. Heating Ammonium Carboxylates
Ammonium salts of carboxylic acids (ammonium carboxylates) on heating, lose a molecule of water to form amides.
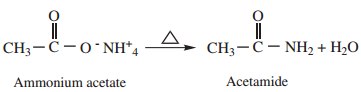
3. Partial Hydrolysis of Alkyl Cyanides (Nitriles)
Partial hydrolysis of alkyl cyanides with cold con HCl gives amides
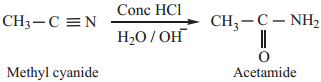
Chemical Properties
1. Amphoteric Character
Amides behave both as weak acid as well as weak base and thus show amphoteric character. This can be proved by the following reactions.
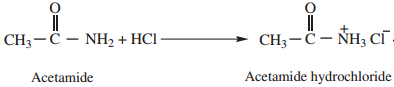
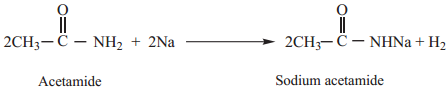
Acetamide (as acid) reacts with sodium to form sodium salt and hydrogen gas is liberated.
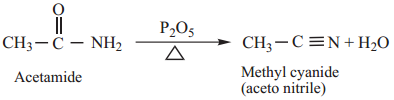
3. Dehydration
Amides on heating with strong dehydrating agents like P2O5 get dehydrated to form cyanides.
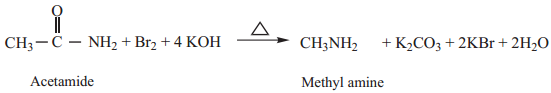
4. Hof Mann’s Degradation
Amides reacts with bromine in the presence of caustic alkali to form a primary amine carrying one carbon less than the parent amide.
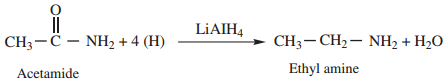
5. Reduction
Amides on reduction with LiAlH4 or Sodium and ethyl alcohol to form corresponding amines.